NPR1 promotes cisplatin resistance by inhibiting PARL-mediated mitophagy-dependent ferroptosis in gastric cancer
- PMID: 39476297
- PMCID: PMC11525271
- DOI: 10.1007/s10565-024-09931-z
NPR1 promotes cisplatin resistance by inhibiting PARL-mediated mitophagy-dependent ferroptosis in gastric cancer
Abstract
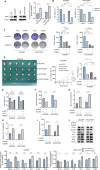
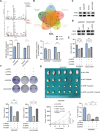
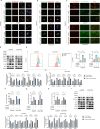
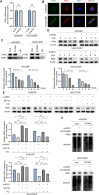
Cisplatin-based chemotherapy serves as the standard of care for individuals with advanced stages of gastric cancer. Nevertheless, the emergence of chemoresistance in GC has detrimental impacts on prognosis, yet the underlying mechanisms governing this phenomenon remain elusive. Level of mitophagy and ferroptosis of GC cells were detected by fluorescence, flow cytometry, GSH, MDA, Fe2+ assays, and to explore the specific molecular mechanisms between NPR1 and cisplatin resistance by performing western blot and coimmunoprecipitation (co-IP) assays. These results indicates that NPR1 positively correlated with cisplatin-resistance and played a crucial part in conferring resistance to cisplatin in gastric cancer cells. Mechanistically, NPR1 affected levels of mitophagy and ferroptosis in human cisplatin-resistance GC cells with cisplatin treatment. Specifically, NPR1 inhibited mitophagy-dependent ferroptosis by reducing the ubiquitination-mediated degradation of PARL; moreover, NPR1 promoted PARL stabilization by disrupting the PARL-MARCH8 complex, which ultimately led to the development of chemoresistance in GC cells. Considering our findings, NPR1 appears to play an important role in chemotherapy for GC. NPR1 could potentially be used to overcome chemotherapy resistance.
Keywords: Chemoresistance; Ferroptosis; Gastric Cancer; Mitophagy; NPR1; PARL; Ubiquitination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ajani JA, et al. Gastric Cancer, Version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–92. 10.6004/jnccn.2022.0008. - PubMed
-
- Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. 10.1038/s41571-020-00462-0. - PubMed
-
- Chen Z, et al. MSC-NPRA loop drives fatty acid oxidation to promote stemness and chemoresistance of gastric cancer. Cancer Lett. 2023;565:216235. 10.1016/j.canlet.2023.216235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

