Aging disrupts blood-brain and blood-spinal cord barrier homeostasis, but does not increase paracellular permeability
- PMID: 39476323
- PMCID: PMC11872845
- DOI: 10.1007/s11357-024-01404-9
Aging disrupts blood-brain and blood-spinal cord barrier homeostasis, but does not increase paracellular permeability
Abstract
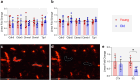
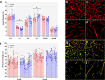
Blood-CNS barriers protect the CNS from circulating immune cells and damaging molecules. It is thought barrier integrity becomes disrupted with aging, contributing to impaired CNS function. Using genome-wide and targeted molecular approaches, we found aging affected expression of predominantly immune invasion and pericyte-related genes in CNS regions investigated, especially after middle age, with spinal cord being most impacted. We did not find significant perturbation of endothelial cell junction genes or proteins, nor were vascular density or pericyte coverage affected by aging. We evaluated barrier paracellular permeability using small molecular weight tracers, serum protein extravasation, CNS water content, and iron labelling measures. We found no evidence for age-related increased barrier permeability in any of these tests. We conclude that blood-brain (BBB) and blood-spinal cord barrier (BSCB) paracellular permeability does not increase with normal aging in mouse. Whilst expression changes were not associated with increased permeability, they may represent an age-related primed state whereby additional insults cause increased leakiness.
Keywords: Aging; Blood–brain barrier; Laser-capture; Proteomics; RNA-Seq; scRNA-seq.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All animal work was undertaken in strict accordance with the University of Newcastle Animal Ethics Committee (protocol numbers: A-2015–526, A-2021–137), and New South Wales and Australian animal research guidelines. Competing interests: The authors declare that they have no competing interests.
Figures





References
-
- Bors L, Toth K, Toth EZ, Bajza A, Csorba A, Szigeti K, et al. Age-dependent changes at the blood-brain barrier. A Comparative structural and functional study in young adult and middle aged rats. Brain Res Bull. 2018;139:269–77. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

