Transcriptomic and metabolic signatures of neural cells cultured under a physiologic-like environment
- PMID: 39476959
- PMCID: PMC11742299
- DOI: 10.1016/j.jbc.2024.107937
Transcriptomic and metabolic signatures of neural cells cultured under a physiologic-like environment
Abstract
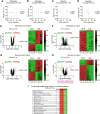
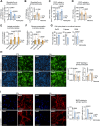
Cultured brain cells are used conventionally to investigate fundamental neurobiology and identify therapeutic targets against neural diseases. However, standard culture conditions do not simulate the natural cell microenvironment, thus hampering in vivo translational insight. Major weaknesses include atmospheric (21%) O2 tension and lack of intercellular communication, the two factors likely impacting metabolism and signaling. Here, we addressed this issue in mouse neurons and astrocytes in primary culture. We found that the signs of cellular and mitochondrial integrity were optimal when these cells were acclimated to grow in coculture, to emulate intercellular coupling, under physiologic (5%) O2 tension. Transcriptomic scrutiny, performed to elucidate the adaptive mechanism involved, revealed that the vast majority of differentially expressed transcripts were downregulated in both astrocytes and neurons. Gene ontology evaluation unveiled that the largest group of altered transcripts was glycolysis, which was experimentally validated by metabolic flux analyses. This protocol and database resource for neural cells grown under in vivo-like microenvironment may move forward the translation of basic into applied neurobiological research.
Keywords: astrocyte; energy metabolism; glycolysis; hypoxia; neuron; transcriptomics.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Keeley T.P., Mann G.E. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol. Rev. 2019;99:161–234. - PubMed
-
- Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23:499–515. - PubMed
-
- Murphy-Royal C., Dupuis J., Groc L., Oliet S.H.R. Astroglial glutamate transporters in the brain: regulating neurotransmitter homeostasis and synaptic transmission. J. Neurosci. Res. 2017;95:2140–2151. - PubMed
-
- Bonvento G., Bolanos J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:1546–1564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

