The GPCR adaptor protein Norbin controls the trafficking of C5aR1 and CXCR4 in mouse neutrophils
- PMID: 39476960
- PMCID: PMC11647504
- DOI: 10.1016/j.jbc.2024.107940
The GPCR adaptor protein Norbin controls the trafficking of C5aR1 and CXCR4 in mouse neutrophils
Abstract
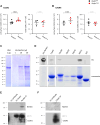
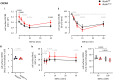
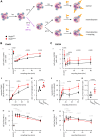
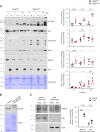
Norbin (Neurochondrin, NCDN) is a G protein-coupled receptor (GPCR) adaptor protein known for its importance in neuronal function. Norbin works by binding to numerous GPCRs, controlling their steady-state trafficking and sometimes their agonist-induced internalization, as well as their signaling. We recently showed that Norbin is expressed in neutrophils, limits the surface levels of the GPCRs C5aR1 and CXCR4 in neutrophils, and suppresses neutrophil-mediated innate immunity. Here, we identify C5aR1 and CXCR4 as direct Norbin interactors and used mice with myeloid-Norbin deficiency to investigate the role of Norbin in the trafficking of endogenous C5aR1 and CXCR4 in primary neutrophils by flow cytometry and cell fractionation. We show that Norbin mediates the agonist-induced internalization of C5aR1 through a β-arrestin-dependent mechanism and limits the recycling of internalized C5aR1 and CXCR4 back to the cell surface. Norbin does not control the constitutive internalization of C5aR1 and CXCR4 nor does it affect the agonist-induced internalization of CXCR4. Norbin suppresses C5aR1 signaling in mouse neutrophils by limiting the C5a-stimulated membrane translocation of Tiam1, Vav, and PKCδ, and activation of Erk and p38 Mapk pathways, as well as Gαi-dependent reactive oxygen species production. Our study demonstrates how Norbin suppresses C5aR1 and CXCR4 function in neutrophils and increases our understanding of the mechanisms through which Norbin regulates GPCR trafficking generally, by identifying its importance in β-arrestin recruitment, β-arrestin dependent agonist-induced receptor internalization, and receptor recycling.
Keywords: C5aR1; CXCR4; G protein–coupled receptor (GPCR); GPCR trafficking; Ncdn; Neurochondrin; P-Rex1; agonist-induced internalization; receptor desensitization; receptor endocytosis; receptor recycling; β-arrestin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Mochizuki R., Ishizuka Y., Yanai K., Koga Y., Fukamizu A. Molecular cloning and expression of human neurochondrin-1 and -2. Biochim. Biophys. Acta. 1999;1446:397–402. - PubMed
-
- Ishizuka Y., Mochizuki R., Yanai K., Takatsuka M., Nonomura T., Niida S., et al. Induction of hydroxyapatite resorptive activity in bone marrow cell populations resistant to bafilomycin A1 by a factor with restricted expression to bone and brain, neurochondrin. Biochim. Biophys. Acta. 1999;1450:92–98. - PubMed
-
- Schwaibold E.M.C., Brandt D.T. Identification of Neurochondrin as a new interaction partner of the FH3 domain of the Diaphanous-related formin Dia1. Biochem. Biophys. Res. Commun. 2008;373:366–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

