The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm
- PMID: 39476963
- PMCID: PMC11629550
- DOI: 10.1016/j.jbc.2024.107932
The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm
Abstract
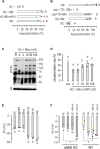
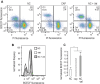
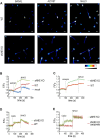
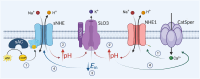
Sperm capacitation is a complex process that takes place in the female reproductive tract and empowers mammalian sperm with the competence to fertilize an egg. It consists of an intricate cascade of events that can be mimicked in vitro through incubation in a medium containing essential components, such as bicarbonate, albumin, Ca2+, and energy substrates, among others. Genetic and pharmacological studies have underscored the unique significance of the K+ channel SLO3 in membrane potential hyperpolarization, as evidenced by the infertility of mice lacking its expression. Notably, two key molecular events, sperm hyperpolarization and intracellular alkalinization, are central to the capacitation process. SLO3 is activated by alkalinization. However, the molecular mechanisms responsible for intracellular alkalization and activation of SLO3 are not completely understood. In this study, we examined the impact of Na+/H+ exchangers (NHEs) on mouse sperm membrane hyperpolarization during capacitation. Pharmacological inhibition of the NHE1 blocked membrane hyperpolarization. A similar effect was observed in sperm deficient of the Ca2+ channel CatSper because of NHE1 not being activated by Ca2+. In addition, the sperm-specific NHE (sNHE) KO did not show membrane hyperpolarization upon capacitation or induction with cAMP analogs. Our results show that sNHE is dually modulated by cAMP and membrane hyperpolarization probably through its cyclic nucleotide-binding domain and the voltage-sensor motif, respectively. Together, sNHE and NHE1 provide the alkalinization need for SLO3 activation during capacitation.
Keywords: adenylate cyclase; cyclic AMP; fertilization; membrane hyperpolarization; potassium channel; sodium–proton exchange; sperm.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Update of
-
The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm.bioRxiv [Preprint]. 2024 Mar 6:2024.03.04.583310. doi: 10.1101/2024.03.04.583310. bioRxiv. 2024. Update in: J Biol Chem. 2024 Dec;300(12):107932. doi: 10.1016/j.jbc.2024.107932. PMID: 38496535 Free PMC article. Updated. Preprint.
References
-
- Stival C., Puga Molina Ldel C., Paudel B., Buffone M.G., Visconti P.E., Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv. Anat. Embryol. Cell Biol. 2016;220:93–106. - PubMed
-
- Matamoros-Volante A., Trevino C.L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci. 2020;133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

