Inflammatory Burden Index Is an Independent Prognostic Factor for Esophageal Cancer Patients Who Receive Curative Treatment
- PMID: 39477415
- PMCID: PMC11535923
- DOI: 10.21873/invivo.13775
Inflammatory Burden Index Is an Independent Prognostic Factor for Esophageal Cancer Patients Who Receive Curative Treatment
Abstract
Background/aim: We hypothesized that the inflammatory burden index (IBI) is a promising biomarker for esophageal cancer (EC) treatment and management. To confirm our hypothesis, we evaluated the prognostic impact of IBI in patients with EC who received curative treatment.
Patients and methods: We conducted a retrospective review of medical records and collected data from consecutive patients with EC who underwent curative resection at Yokohama City University between 2005 and 2020. The IBI score was calculated as the C-reactive protein level multiplied by the neutrophil-to-lymphocyte ratio.
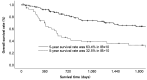
Results: In total, 180 patients with EC were included in this study. The 3- and 5-year overall survival (OS) rates were 72.9% and 63.4%, respectively, in the IBI-low group, and 38.2% and 32.5% in the IBI-high group (p<0.001). In the multivariate analysis, IBI was identified as a significant prognostic factor for OS [hazard ratio (HR)=2.372; 95% confidence interval CI=1.478-3.806, p<0.001]. In addition, the 3- and 5-year recurrence-free survival (RFS) rates were 52.9% and 47.8%, respectively, in the IBI-low group, and 22.9% and 17.2% in the IBI-high group (p<0.001). In the multivariate analysis, IBI was identified as a significant prognostic factor for RFS (HR=2.484; 95%CI=1.373-4.494, p<0.001). When comparing the recurrence patterns between the IBI-high and IBI-low groups, there were significant differences in lymph node recurrence (46.0% vs. 26.2%, p=0.010) and hematological recurrence (52.0% vs. 18.5%, p<0.001).
Conclusion: IBI affects both the short- and long-term oncological outcomes. Thus, IBI may be a promising prognostic factor for the treatment and management of EC.
Keywords: Inflammatory burden index; esophageal cancer; survival.
Copyright © 2024, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest in association with the present study.
Figures
References
-
- Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20(3):343–372. doi: 10.1007/s10388-023-00993-2. - DOI - PMC - PubMed
-
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials