Association Between Multisystem Immune-related Adverse Events and Progression-free Survivals in PD-1/PD-L1 Inhibitor Monotherapy
- PMID: 39477436
- PMCID: PMC11535954
- DOI: 10.21873/invivo.13770
Association Between Multisystem Immune-related Adverse Events and Progression-free Survivals in PD-1/PD-L1 Inhibitor Monotherapy
Abstract
Background/aim: Immune-related adverse events (irAEs) occur in various organs, and sometimes multiply following treatment with immune checkpoint inhibitors (ICIs). This study aimed to determine the association between the number of irAEs and clinical outcomes.
Patients and methods: This was a retrospective study that included patients with lung cancer, melanoma, and head and neck cancer who were treated with anti-programmed cell death (ligand) 1 (PD-1/PD-L1) monotherapy. We evaluated the association between the number of irAEs and progression-free survival (PFS) in the simple Cox regression analysis. To eliminate the immortal-time bias, an additional landmark analysis was performed.
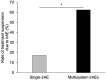
Results: In total, 92, 69, and 37 patients were allocated to the no, single, and multisystem irAEs groups, respectively. The multisystem irAEs were associated with better PFS compared to the no irAE group. In contrast, at the 12-week landmark, multisystem irAEs were associated with poor PFS compared to the no irAEs group. Furthermore, the rate of treatment suspension owing to irAEs in the multisystem irAEs group (62.5%) was higher than that in the single irAE group (17.3%) at the 12-week landmark.
Conclusion: The incidence of multisystem irAEs was associated with improved clinical outcomes in patients with lung cancer, melanoma, and head and neck cancer treated with PD-1/PD-L1 inhibitor monotherapy. However, these results may be influenced by a potential immortal-time bias. When accounting for this bias, the early development of multisystem irAEs within 12 weeks was linked to treatment suspension and poorer clinical outcomes.
Keywords: Immune checkpoint inhibitors; efficacy; immortal-time bias; immune-related adverse events; multisystem irAEs; progression-free survival.
Copyright © 2024, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare that there are no competing interests in relation to this study.
Figures




References
-
- Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–857. doi: 10.1158/2159-8290.CD-20-1680. - DOI - PubMed
-
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, Davies MJ, Ernstoff MS, Fecher L, Ghosh M, Jaiyesimi I, Mammen JS, Naing A, Nastoupil LJ, Phillips T, Porter LD, Reichner CA, Seigel C, Song JM, Spira A, Suarez-Almazor M, Swami U, Thompson JA, Vikas P, Wang Y, Weber JS, Funchain P, Bollin K. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
