Incorporation of photosynthetically active algal chloroplasts in cultured mammalian cells towards photosynthesis in animals
- PMID: 39477444
- PMCID: PMC11635087
- DOI: 10.2183/pjab.100.035
Incorporation of photosynthetically active algal chloroplasts in cultured mammalian cells towards photosynthesis in animals
Abstract
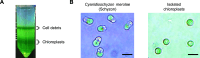
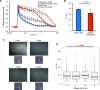
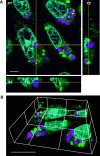
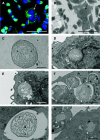
Chloroplasts are photosynthetic organelles that evolved through the endosymbiosis between cyanobacteria-like symbionts and hosts. Many studies have attempted to isolate intact chloroplasts to analyze their morphological characteristics and photosynthetic activity. Although several studies introduced isolated chloroplasts into the cells of different species, their photosynthetic activities have not been confirmed. In this study, we isolated photosynthetically active chloroplasts from the primitive red alga Cyanidioschyzon merolae and incorporated them in cultured mammalian cells via co-cultivation. The incorporated chloroplasts retained their thylakoid structure in intracellular vesicles and were maintained in the cytoplasm, surrounded by the mitochondria near the nucleus. Moreover, the incorporated chloroplasts maintained electron transport activity of photosystem II in cultured mammalian cells for at least 2 days after the incorporation. Our top-down synthetic biology-based approach may serve as a foundation for creating artificially photosynthetic animal cells.
Keywords: algae; chloroplast; cultured mammalian cell; photosynthesis; synthetic biology.
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Matsunaga S. (2018) Planimal cells: Artificial photosynthetic animal cells inspired by endosymbiosis and photosynthetic animals. Cytologia 83, 3–6.

