Outcomes for Patients with Metastatic Castration-Resistant Prostate Cancer and Liver Metastasis Receiving [177Lu]Lu-PSMA-617
- PMID: 39477495
- PMCID: PMC11619589
- DOI: 10.2967/jnumed.124.268277
Outcomes for Patients with Metastatic Castration-Resistant Prostate Cancer and Liver Metastasis Receiving [177Lu]Lu-PSMA-617
Abstract
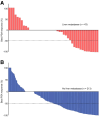
It is well known that patients with liver metastasis from metastatic castration-resistant prostate cancer have poor or only transient responses to many forms of systemic therapy. Data on outcomes after treatment with [177Lu]Lu-PSMA-617 (LuPSMA) are scarce. The VISION trial reports a hazard ratio for overall survival (OS) in the subgroup of patients with liver metastasis without disclosing the absolute duration of survival. Using real-world clinical data, we examined this important subgroup of patients, describing prostate-specific antigen (PSA) response and OS. Methods: A single-institution database was assembled to include all patients receiving LuPSMA at Mayo Clinic in Rochester, Minnesota, for whom treatment was initiated between March 2022 and March 2023. Baseline clinicopathologic and imaging characteristics were abstracted. Patients were then categorized by presence or absence of liver metastasis on pretreatment prostate-specific membrane antigen (PSMA) PET. PSA response and OS for the 2 groups (liver metastasis vs. no liver metastasis) were compared using χ2 testing and the Kaplan-Meier method, respectively. A multivariate Cox regression analysis was performed, including established prognostic factors. Finally, those with pretreatment circulating tumor DNA as determined in an 83-gene panel were assessed for the presence of pathogenic and likely pathogenic alterations. These findings were summarized using descriptive statistics and compared between the 2 cohorts using the Fisher exact test. Results: The overall cohort consisted of 273 patients, including 43 (15.75%) with liver metastasis on pretreatment PSMA PET/CT. The median number of cycles received was 3 (range, 1-6) for patients with liver metastasis and 5 (range, 1-6) for those without hepatic involvement. The 50% or greater reduction in PSA from baseline response rate was lower for those with liver metastasis than for those without (30.23% [13/43] vs 49.77% [106/213], P = 0.019). At a median follow-up of 10 mo (interquartile range, 9-13 mo), there was a significant difference in median OS (8.35 mo vs. not reached, P < 0.001). On multivariate analysis, the presence of liver metastasis was independently associated with shorter survival (hazard ratio, 4.06; P < 0.001). Conclusion: Our data suggest that the presence of liver metastasis predicts poorer outcomes in patients receiving LuPSMA treatment. Alternative and combination approaches should be explored to maximize the antitumor activity of radiopharmaceutical therapy in the liver.
Keywords: LuPSMA; PSMA-targeted radiopharmaceuticals; [177Lu]Lu-PSMA-617; liver metastases; mCRPC; metastatic castration-resistant prostate cancer.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
References
-
- Tsilimigras DI, Brodt P, Clavien P-A, et al. Liver metastases. Nat Rev Dis Primers. 2021;7:27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous