Kinetic Analysis and Metabolism of Poly(Adenosine Diphosphate-Ribose) Polymerase-1-Targeted 18F-Fluorthanatrace PET in Breast Cancer
- PMID: 39477499
- PMCID: PMC11619586
- DOI: 10.2967/jnumed.124.268254
Kinetic Analysis and Metabolism of Poly(Adenosine Diphosphate-Ribose) Polymerase-1-Targeted 18F-Fluorthanatrace PET in Breast Cancer
Abstract
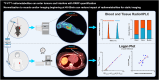
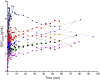
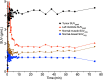
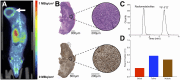
The poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPi) have demonstrated efficacy in ovarian, breast, and prostate cancers, but current biomarkers do not consistently predict clinical benefit. 18F-fluorthanatrace (18F-FTT) is an analog to rucaparib, a clinically approved PARPi, and is a candidate biomarker for PARPi response. This study intends to characterize 18F-FTT pharmacokinetics in breast cancer and optimize image timing for clinical trials. A secondary aim is to determine whether 18F-FTT uptake in breast cancer correlates with matched frozen surgical specimens as a reference standard for PARP-1 protein. Methods: Thirty prospectively enrolled women with a new diagnosis of breast cancer were injected with 18F-FTT and imaged dynamically 0-60 min after injection over the chest, with an optional static scan over multiple bed positions starting around 70 min. Kinetic analysis of lesion uptake was performed using blood-pool activity with population radiometabolite corrections. Normal breast and normal muscle reference tissue models were compared with PARP-1 protein expression in 10 patients with available tissue. Plasma radiometabolite concentrations and uptake in tumor and normal muscle were investigated in mouse xenografts. Results: Pharmacokinetics of 18F-FTT were well fit by Logan plot reference region models of reversible binding. However, fits of 2-tissue compartment models assuming negligible metabolite uptake were unstable. Rapid metabolism of 18F-FTT was demonstrated in mice, and similar uptake of radiometabolites was found in tumor xenografts and normal muscle. Tumor 18F-FTT distribution volume ratios relative to normal muscle reference tissue correlated with tissue PARP-1 expression (P < 0.02, n = 10). The tumor-to-normal muscle ratio from a 5-min frame between 50 and 60 min after injection, a potential static scan protocol, closely corresponded to the distribution volume ratio relative to normal muscle and correlated to PARP-1 expression (P < 0.02, n = 10). Conclusion: This study of PARPi analog 18F-FTT showed that uptake kinetics in vivo corresponded to expression of PARP-1 and that 18F-FTT quantitation is influenced by radiometabolites that are increasingly present late after injection. Radiometabolites can be controlled by using optimal image acquisition timing or normal muscle reference tissue modeling in dynamic imaging or a tumor-to-normal muscle ratio. Optimal image timing for tumor-to-normal muscle quantification in humans appears to be between 50 and 60 min after injection. Therefore, a clinically practical static imaging protocol commencing 45-55 min after injection may sufficiently balance 18F-FTT uptake with background clearance and radiometabolite interference for quantitative interpretation of PARP-1 expression in vivo.
Keywords: 18F-fluorthanatrace; PARP-1; breast cancer; radiotracer tissue pharmacokinetics.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
-
- McCabe N, Turner NC, Lord CJ, et al. . Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
