The Critical Thing about the Ear's Sensory Hair Cells
- PMID: 39477536
- PMCID: PMC11529813
- DOI: 10.1523/JNEUROSCI.1583-24.2024
The Critical Thing about the Ear's Sensory Hair Cells
Abstract
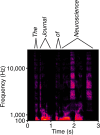
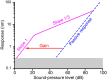
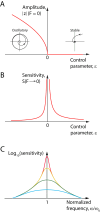
The capabilities of the human ear are remarkable. We can normally detect acoustic stimuli down to a threshold sound-pressure level of 0 dB (decibels) at the entrance to the external ear, which elicits eardrum vibrations in the picometer range. From this threshold up to the onset of pain, 120 dB, our ears can encompass sounds that differ in power by a trillionfold. The comprehension of speech and enjoyment of music result from our ability to distinguish between tones that differ in frequency by only 0.2%. All these capabilities vanish upon damage to the ear's receptors, the mechanoreceptive sensory hair cells. Each cochlea, the auditory organ of the inner ear, contains some 16,000 such cells that are frequency-tuned between ∼20 Hz (cycles per second) and 20,000 Hz. Remarkably enough, hair cells do not simply capture sound energy: they can also exhibit an active process whereby sound signals are amplified, tuned, and scaled. This article describes the active process in detail and offers evidence that its striking features emerge from the operation of hair cells on the brink of an oscillatory instability-one example of the critical phenomena that are widespread in physics.
Keywords: auditory system; cochlea; gating spring; hair bundle; transduction; vestibular system.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Adachi N, Yoshida T, Nin F, Ogata G, Yamaguchi S, Suzuki T, Komune S, Hisa Y, Hibino H, Kurachi Y (2013) The mechanism underlying maintenance of the endocochlear potential by the K+ transport system in fibrocytes of the inner ear. J Physiol 591:4459–4472. 10.1113/jphysiol.2013.258046 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
