Effect of fenofibrate on residual beta cell function in adults and adolescents with newly diagnosed type 1 diabetes: a randomised clinical trial
- PMID: 39477880
- PMCID: PMC11663161
- DOI: 10.1007/s00125-024-06290-6
Effect of fenofibrate on residual beta cell function in adults and adolescents with newly diagnosed type 1 diabetes: a randomised clinical trial
Abstract
Aims/hypothesis: Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, shows some promise in alleviating beta cell stress and preserving beta cell function in preclinical studies of type 1 diabetes. The aim of this phase 2, placebo-controlled, double-blinded, randomised clinical trial was to investigate the efficacy and safety of fenofibrate in adults and adolescents with newly diagnosed type 1 diabetes.
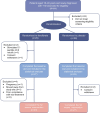
Methods: We enrolled 58 individuals (aged 16 to 40 years old) with newly diagnosed type 1 diabetes and randomised them to daily oral treatment with fenofibrate 160 mg or placebo for 52 weeks (in a block design with a block size of 4, assigned in a 1:1 ratio). Our primary outcome was change in beta cell function after 52 weeks of treatment, assessed by AUC for C-peptide levels following a 2 h mixed-meal tolerance test. Secondary outcomes included glycaemic control (assessed by HbA1c and continuous glucose monitoring), daily insulin use, and proinsulin/C-peptide (PI/C) ratio as a marker of beta cell stress. We assessed outcome measures before and after 4, 12, 26 and 52 weeks of treatment. Blinding was maintained for participants, their healthcare providers and all staff involved in handling outcome samples and assessment.
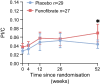
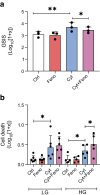
Results: The statistical analyses for the primary outcome included 56 participants (n=27 in the fenofibrate group, after two withdrawals, and n=29 in the placebo group). We found no significant differences between the groups in either 2 h C-peptide levels (mean difference of 0.08 nmol/l [95% CI -0.05, 0.23]), insulin use or glycaemic control after 52 weeks of treatment. On the contrary, the fenofibrate group showed a higher PI/C ratio at week 52 compared with placebo (mean difference of 0.024 [95% CI 0.000, 0.048], p<0.05). Blood lipidome analysis revealed that fenofibrate repressed pathways involved in sphingolipid metabolism and signalling at week 52 compared with placebo. The 52 week intervention evoked few adverse events and no serious adverse events. Follow-up in vitro experiments in human pancreatic islets demonstrated a stress-inducing effect of fenofibrate.
Conclusions/interpretation: Contrary to the beneficial effects of fenofibrate found in preclinical studies, this longitudinal, randomised, placebo-controlled trial does not support the use of fenofibrate for preserving beta cell function in individuals with newly diagnosed type 1 diabetes.
Trial registration: EudraCT number: 2019-004434-41 FUNDING: This study was funded by the Sehested Hansens Foundation.
Keywords: Beta cell function; Beta cell stress; Clinical trial; Fenofibrate; Newly diagnosed type 1 diabetes; Peroxisome proliferator-activated receptor alpha (PPARα); Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Acknowledgements: We thank all participants and study project members for their participation in the study, and J. Rungby (Steno Diabetes Center Copenhagen) and D. Vistisen (Steno Diabetes Center Copenhagen) for serving on the data monitoring committee. Data availability: Data supporting the results from this study are available from the corresponding author on reasonable request. Funding: This study was funded by the Sehested Hansens Foundation. Authors’ relationships and activities: All authors declare that they have no relationships or activities that might bias, or be perceived to bias, their contribution to this study. Contribution statement: All authors contributed to and approved the final version of the manuscript. PEH drafted the trial protocol, collected clinical data, performed the final statistical analyses, and wrote the first draft of the manuscript. TS and SBH made substantial contributions to the data collection process. JJ, HUA, KB, YH and FP were instrumental in the conception and design of the study. Additionally, they provided valuable feedback and engaged in intellectual discussions. RHG and JS designed and performed the human islets experiments. KS and MH performed lipidomic analysis and bioinformatics, respectively. As the principal investigator, FP provided supervision and guidance throughout all stages of this work. FP is the guarantor and takes full responsibility for the integrity and accuracy of this work.
Figures



References
-
- Buschard K, Blomqvist M, Mansson JE, Fredman P, Juhl K, Gromada J (2006) C16:0 sulfatide inhibits insulin secretion in rat beta-cells by reducing the sensitivity of KATP channels to ATP inhibition. Diabetes 55(10):2826–2834. 10.2337/db05-1355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

