Risk-stratified treatment for drug-susceptible pulmonary tuberculosis
- PMID: 39477924
- PMCID: PMC11526018
- DOI: 10.1038/s41467-024-53273-7
Risk-stratified treatment for drug-susceptible pulmonary tuberculosis
Erratum in
-
Author Correction: Risk-stratified treatment for drug-susceptible pulmonary tuberculosis.Nat Commun. 2025 May 13;16(1):4438. doi: 10.1038/s41467-025-59791-2. Nat Commun. 2025. PMID: 40360559 Free PMC article. No abstract available.
Abstract
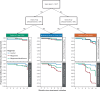
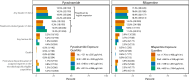
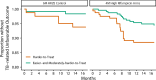
The Phase 3 randomized controlled trial, TBTC Study 31/ACTG A5349 (NCT02410772) demonstrated that a 4-month rifapentine-moxifloxacin regimen for drug-susceptible pulmonary tuberculosis was safe and effective. The primary efficacy outcome was 12-month tuberculosis disease free survival, while the primary safety outcome was the proportion of grade 3 or higher adverse events during the treatment period. We conducted an analysis of demographic, clinical, microbiologic, radiographic, and pharmacokinetic data and identified risk factors for unfavorable outcomes and adverse events. Among participants receiving the rifapentine-moxifloxacin regimen, low rifapentine exposure is the strongest driver of tuberculosis-related unfavorable outcomes (HR 0.65 for every 100 µg∙h/mL increase, 95%CI 0.54-0.77). The only other risk factors identified are markers of higher baseline disease severity, namely Xpert MTB/RIF cycle threshold and extent of disease on baseline chest radiography (Xpert: HR 1.43 for every 3-cycle-threshold decrease, 95%CI 1.07-1.91; extensive disease: HR 2.02, 95%CI 1.07-3.82). From these risk factors, we developed a simple risk stratification to classify disease phenotypes as easier-, moderately-harder, or harder-to-treat TB. Notably, high rifapentine exposures are not associated with any predefined adverse safety outcomes. Our results suggest that the easier-to-treat subgroup may be eligible for further treatment shortening while the harder-to-treat subgroup may need higher doses or longer treatment.
© 2024. The Author(s).
Conflict of interest statement
The authorship team members have declared no potential conflicts of interest with respect to the research, authorship, or publication of this article. Sanofi commercial interests did not influence the study design; the collection, analysis, or interpretation of data; the preparation of this manuscript; or the decision to submit this manuscript for publication. A Sanofi technical expert served on the protocol team.
Figures


References
-
- World Health Organization. Treatment of Drug-Susceptible Tuberculosis: Rapid Communication. (World Health Organization, Geneva, 2021).
-
- WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-susceptible tuberculosis treatment. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. - PubMed

