Post-acute sequelae of SARS-CoV-2 cardiovascular symptoms are associated with trace-level cytokines that affect cardiomyocyte function
- PMID: 39478108
- PMCID: PMC11602718
- DOI: 10.1038/s41564-024-01838-z
Post-acute sequelae of SARS-CoV-2 cardiovascular symptoms are associated with trace-level cytokines that affect cardiomyocyte function
Abstract
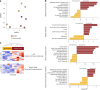
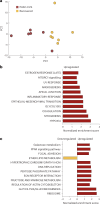
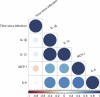
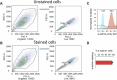
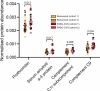
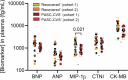
An estimated 65 million people globally suffer from post-acute sequelae of COVID-19 (PASC), with many experiencing cardiovascular symptoms (PASC-CVS) like chest pain and heart palpitations. This study examines the role of chronic inflammation in PASC-CVS, particularly in individuals with symptoms persisting over a year after infection. Blood samples from three groups-recovered individuals, those with prolonged PASC-CVS and SARS-CoV-2-negative individuals-revealed that those with PASC-CVS had a blood signature linked to inflammation. Trace-level pro-inflammatory cytokines were detected in the plasma from donors with PASC-CVS 18 months post infection using nanotechnology. Importantly, these trace-level cytokines affected the function of primary human cardiomyocytes. Plasma proteomics also demonstrated higher levels of complement and coagulation proteins in the plasma from patients with PASC-CVS. This study highlights chronic inflammation's role in the symptoms of PASC-CVS.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: K.R.S. has historically been a consultant for Sanofi, Pfizer, Roche and NovoNordisk. The opinions and data presented in this manuscript are of the authors and are independent of these relationships. The remaining authors declare no competing interests.
Figures









References
-
- Short, K. R. Long COVID. Microbiol. Aust.44, 113–114 (2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

