Rhythmic IL-17 production by γδ T cells maintains adipose de novo lipogenesis
- PMID: 39478228
- PMCID: PMC11618085
- DOI: 10.1038/s41586-024-08131-3
Rhythmic IL-17 production by γδ T cells maintains adipose de novo lipogenesis
Abstract
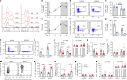
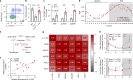
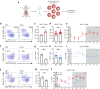
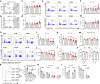
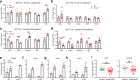
The circadian rhythm of the immune system helps to protect against pathogens1-3; however, the role of circadian rhythms in immune homeostasis is less well understood. Innate T cells are tissue-resident lymphocytes with key roles in tissue homeostasis4-7. Here we use single-cell RNA sequencing, a molecular-clock reporter and genetic manipulations to show that innate IL-17-producing T cells-including γδ T cells, invariant natural killer T cells and mucosal-associated invariant T cells-are enriched for molecular-clock genes compared with their IFNγ-producing counterparts. We reveal that IL-17-producing γδ (γδ17) T cells, in particular, rely on the molecular clock to maintain adipose tissue homeostasis, and exhibit a robust circadian rhythm for RORγt and IL-17A across adipose depots, which peaks at night. In mice, loss of the molecular clock in the CD45 compartment (Bmal1∆Vav1) affects the production of IL-17 by adipose γδ17 T cells, but not cytokine production by αβ or IFNγ-producing γδ (γδIFNγ) T cells. Circadian IL-17 is essential for de novo lipogenesis in adipose tissue, and mice with an adipocyte-specific deficiency in IL-17 receptor C (IL-17RC) have defects in de novo lipogenesis. Whole-body metabolic analysis in vivo shows that Il17a-/-Il17f-/- mice (which lack expression of IL-17A and IL-17F) have defects in their circadian rhythm for de novo lipogenesis, which results in disruptions to their whole-body metabolic rhythm and core-body-temperature rhythm. This study identifies a crucial role for IL-17 in whole-body metabolic homeostasis and shows that de novo lipogenesis is a major target of IL-17.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





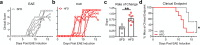


References
-
- Scheiermann, C., Gibbs, J., Ince, L. & Loudon, A. Clocking in to immunity. Nat. Rev. Immunol.18, 423–437 (2018). - PubMed
-
- Trim, W. V. & Lynch, L. Immune and non-immune functions of adipose tissue leukocytes. Nat. Rev. Immunol.22, 371–386 (2022). - PubMed
-
- Kane, H. & Lynch, L. Innate immune control of adipose tissue homeostasis. Trends Immunol.40, 857–872 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

