Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei
- PMID: 39478231
- PMCID: PMC11634766
- DOI: 10.1038/s41586-024-08151-z
Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei
Erratum in
-
Publisher Correction: Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei.Nature. 2024 Dec;636(8042):E3. doi: 10.1038/s41586-024-08389-7. Nature. 2024. PMID: 39550527 Free PMC article. No abstract available.
Abstract
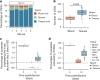
The protozoan parasite Trypanosoma brucei evades clearance by the host immune system through antigenic variation of its dense variant surface glycoprotein (VSG) coat, periodically 'switching' expression of the VSG using a large genomic repertoire of VSG-encoding genes1-6. Recent studies of antigenic variation in vivo have focused near exclusively on parasites in the bloodstream6-8, but research has shown that many, if not most, parasites reside in the interstitial spaces of tissues9-13. We sought to explore the dynamics of antigenic variation in extravascular parasite populations using VSG-seq7, a high-throughput sequencing approach for profiling VSGs expressed in populations of T. brucei. Here we show that tissues, not the blood, are the primary reservoir of antigenic diversity during both needle- and tsetse bite-initiated T. brucei infections, with more than 75% of VSGs found exclusively within extravascular spaces. We found that this increased diversity is correlated with slower parasite clearance in tissue spaces. Together, these data support a model in which the slower immune response in extravascular spaces provides more time to generate the antigenic diversity needed to maintain a chronic infection. Our findings reveal the important role that extravascular spaces can have in pathogen diversification.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Cross, G. A. M., Kim, H. S. & Wickstead, B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol. Biochem. Parasitol.195, 59–73 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

