Efficacy of Treatments in Reducing Facial Erythema in Rosacea: A Systematic Review
- PMID: 39478371
- PMCID: PMC11829502
- DOI: 10.1177/12034754241287546
Efficacy of Treatments in Reducing Facial Erythema in Rosacea: A Systematic Review
Abstract
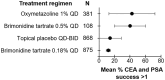
Rosacea is a chronic inflammatory skin condition that affects over 5% of individuals worldwide. Its clinical presentation is characterized by an array of features, including erythema, papules and pustules, phymatous changes, telangiectasia, and ocular manifestations. Specifically, the multifaceted manifestation of erythema varies widely in intensity and distribution. Factors contributing to pathogenesis include neurovascular dysregulation, increased levels of pro-inflammatory mediators, and aberrant vasodilation. Erythema management plays an important role in reducing the psychosocial burden associated with rosacea and improving overall quality of life. Cochrane CENTRAL, Medline, and Embase databases were searched from inception to September 2023 and included 33 clinical trials reporting on a total of 7411 rosacea patients (74.1% female) and 21 different topical or systemic treatments. The mean age was 48.8 years (range, 18-83 years), and the mean time to outcome assessment was 8.1 weeks (standard deviation, 4.1 weeks). Treatment efficacy was assessed by outcome measures including percent improvement from baseline on 4- and 5-point scales, clinician erythema assessment (CEA) success (improvement ≥1 point), and CEA and patient self-assessment success (improvement ≥1 point). Pooled effect sizes for each treatment were calculated as a weighted average based on the number of patients in each study. The most effective topical treatments for reducing erythema include sodium sulphacetamide and sulphur, praziquantel, metronidazole, and B244 spray (Nitrosomonas eutropha). The most effective systemic treatment was paroxetine. Our findings highlight the varying efficacy of treatments in addressing the erythema in rosacea, recognizing the nuances of clinical presentations.
Keywords: erythema; rosacea; treatment.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CS has received honoraria from Abbvie, Leo, Pfizer, Miravo, Novartis, UCB, Sanofi/Regeneron unrelated to this work. RGS has received honoraria from Perfuse, Quart Medical, Novartis, Medexus Pharmaceuticals Canada along with Ontario Gov’t (Project ECHO Ontario Skin & Wound-Ministry of Health and Micro-credentials—through Ministry of Colleges and Universities and Sault College) all unrelated to this work. NJH, JC, and RSQG have no conflicts of interest to disclose.
Figures



References
-
- Farshchian M, Daveluy S. Rosacea. In: StatPearls. StatPearls Publishing; 2023:1-2. http://www.ncbi.nlm.nih.gov/books/NBK557574/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

