Predictors of abemaciclib discontinuation in patients with breast cancer: a multicenter retrospective cohort study
- PMID: 39478497
- PMCID: PMC11523900
- DOI: 10.1186/s12885-024-13091-y
Predictors of abemaciclib discontinuation in patients with breast cancer: a multicenter retrospective cohort study
Abstract
Objective: This study explored the predictors of abemaciclib discontinuation, a cyclin-dependent kinase 4 and 6 inhibitor, in patients with breast cancer.
Material and methods: Between November 2018 and March 2023, 147 patients with breast cancer treated with abemaciclib at Osaka Medical and Pharmaceutical University Hospital and Kindai University Nara Hospital were included. The exclusion criteria were as follows: lack of blood testing within 2 weeks prior to starting abemaciclib therapy, transfer to another facility after the commencement of abemaciclib therapy, and discontinuation of abemaciclib therapy due to the diagnosis of another cancer. The duration from the initiation of abemaciclib to discontinuation for any reason and to temporary suspension or dose reduction due to adverse events were analyzed as outcome variables using multivariate Cox regression analysis.
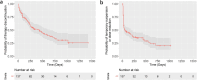
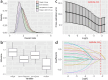
Results: Baseline weight < 54 kg, bone metastases, and hemoglobin level ≤ 12.4 g/dL were independent predictors of abemaciclib discontinuation for any reason. The main adverse events leading to abemaciclib discontinuation were liver enzyme elevation and gastrointestinal symptoms. Additionally, focusing on the adverse event of abemaciclib, a baseline weight < 54 kg was an independent predictor of temporary suspension or dose reduction due to adverse events. The most common adverse events leading to temporary suspension or dose reduction were neutropenia and diarrhea.
Conclusion: Patients with lower body weight are more susceptible to the adverse events of abemaciclib, increasing their risk of treatment discontinuation. In such patients, strict monitoring of adverse events and consideration of more frequent medical visits are necessary from the start of abemaciclib therapy.
Keywords: Abemaciclib; Breast cancer; Chemotherapy; Discontinuation; Predictor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Pedersen RN, Mellemkjær L, Ejlertsen B, et al. Mortality after late breast cancer recurrence in Denmark. J Clin Oncol. 2022;40(13):1450. - PubMed
-
- Sambi M, Qorri B, Harless W, Szewczuk MR. Therapeutic options for metastatic breast cancer. Adv Exp Med Biol. 2019;1152:131. - PubMed
-
- O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

