Low-dose dobutamine in acute myocardial infarction with intermediate to high risk of cardiogenic shock development (the DOBERMANN-D trial): study protocol for a double-blinded, placebo-controlled, single-center, randomized clinical trial
- PMID: 39478521
- PMCID: PMC11523592
- DOI: 10.1186/s13063-024-08567-y
Low-dose dobutamine in acute myocardial infarction with intermediate to high risk of cardiogenic shock development (the DOBERMANN-D trial): study protocol for a double-blinded, placebo-controlled, single-center, randomized clinical trial
Abstract
Background: Cardiogenic shock (CS) occurs in 5-10% of patients with acute myocardial infarction (AMI), and the condition is associated with a 30-day mortality rate of up to 50%. Most of the AMI patients are in SCAI SHOCK stage B upon hospital arrival, but some of these patients will progression through the stages to overt shock (SCAI C-E). Around one third of patients who develop CS are not in shock at the time of hospital admission. Pro-B-type natriuretic peptide (proband) is a biomarker closely related to CS development. The aim of this study is to investigate the potential for preventing progression of hemodynamic instability by early inotropic support with low-dose dobutamine infusion administrated after revascularization in AMI patients with intermediate to high risk of in-hospital CS development.
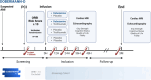
Methods: This investigator-initiated, double-blinded, placebo-controlled, randomized, single-center, clinical trial will include 100 AMI patients (≥ 18 years) without CS at hospital admission and at intermediate-high risk of in-hospital CS development (ORBI risk score ≥ 10). Patients will be randomized in a 1:1 ratio to a 24 h intravenous (IV) infusion of dobutamine (5 μg/kg/min) or placebo (NaCl) administrated after acute percutaneous coronary intervention (PCI) (< 24 h from symptom onset). Blood samples are drawn at time points from study inclusion (before infusion, 12, 24, 36, and 48 h). The primary outcome is peak plasma proBNP within 48 h after infusion as a surrogate-measure for the hemodynamic status. Hemodynamic function will be assessed pulse rate, blood pressure, and lactate within 48 h after infusion and by transthoracic echocardiography (TTE) performed after 24-48 h and at follow-up after 3 months. Markers of cardiac injury (troponin T and creatine kinase MB (CK-MB)) will be assessed.
Discussion: Early inotropic support with low-dose dobutamine infusion in patients with AMI, treated with acute PCI, and at intermediate-high risk of in-hospital CS may serve as an intervention promoting hemodynamic stability and facilitating patient recovery. The effect will be assessed using proBNP as a surrogate marker of CS development, hemodynamic measurements, and TTE within the initial 48 h and repeated at a 3-month follow-up.
Trial registration: The Regional Ethics Committee : H-21045751. EudraCT: 2021-002028-19.
Clinicaltrials: gov: NCT05350592, Registration date: 2022-03-08. WHO Universal Trial Number: U1111-1277-8523.
Keywords: Acute myocardial infarction; Cardiogenic shock; Dobutamine; Hemodynamic; Inotropy; Neurohormonal activation; ORBI risk score; Percutaneous coronary intervention; Transthoracic echocardiography.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Interleukin-6 receptor antibodies (tocilizumab) in acute myocardial infarction with intermediate to high risk of cardiogenic shock development (DOBERMANN-T): study protocol for a double-blinded, placebo-controlled, single-center, randomized clinical trial.Trials. 2024 Nov 5;25(1):739. doi: 10.1186/s13063-024-08573-0. Trials. 2024. PMID: 39501388 Free PMC article.
-
Randomized Control of Sympathetic Drive With Continuous Intravenous Esmolol in Patients With Acute ST-Segment Elevation Myocardial Infarction: The BEtA-Blocker Therapy in Acute Myocardial Infarction (BEAT-AMI) Trial.JACC Cardiovasc Interv. 2016 Feb 8;9(3):231-240. doi: 10.1016/j.jcin.2015.10.035. JACC Cardiovasc Interv. 2016. PMID: 26847114 Clinical Trial.
-
Prognostic effect of sex according to shock severity in patients with acute myocardial infarction complicated by cardiogenic shock.Hellenic J Cardiol. 2025 Mar-Apr;82:3-14. doi: 10.1016/j.hjc.2023.11.007. Epub 2023 Dec 10. Hellenic J Cardiol. 2025. PMID: 38072307
-
Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome.Cochrane Database Syst Rev. 2020 Nov 5;11(11):CD009669. doi: 10.1002/14651858.CD009669.pub4. Cochrane Database Syst Rev. 2020. PMID: 33152122 Free PMC article.
-
Current clinical management of acute myocardial infarction complicated by cardiogenic shock.Expert Rev Cardiovasc Ther. 2021 Jan;19(1):41-46. doi: 10.1080/14779072.2021.1854733. Epub 2021 Jan 19. Expert Rev Cardiovasc Ther. 2021. PMID: 33289436 Review.
Cited by
-
Prognostic value of lactate clearance, fluid balance, and APACHE II score in patients with cardiogenic shock receiving extracorporeal membrane oxygenation.Front Cardiovasc Med. 2025 May 7;12:1557909. doi: 10.3389/fcvm.2025.1557909. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40401225 Free PMC article.
References
-
- Christensen DM, Schjerning AM, Smedegaard L, Charlot MG, Ravn PB, Ruwald AC, et al. Long-term mortality, cardiovascular events, and bleeding in stable patients 1 year after myocardial infarction: a Danish nationwide study. Eur Heart J. 2023;44:488–98. 10.1093/eurheartj/ehac667. Cited 2024 Jun 13. - PMC - PubMed
-
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–97. - PubMed
-
- Obling L, Frydland M, Hansen R, Møller-Helgestad OK, Lindholm MG, Holmvang L, et al. Risk factors of late cardiogenic shock and mortality in ST-segment elevation myocardial infarction patients. Eur Heart J Acute Cardiovasc Care. 2018;7(1):7–15. - PubMed
-
- De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur J Heart Fail. 2015;17(11):1124–32. - PubMed
-
- Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

