Quantitative micro-CT-derived biomarkers elucidate age-related lung fibrosis in elder mice
- PMID: 39478545
- PMCID: PMC11526612
- DOI: 10.1186/s12931-024-03006-7
Quantitative micro-CT-derived biomarkers elucidate age-related lung fibrosis in elder mice
Abstract
Background: Idiopathic Pulmonary Fibrosis (IPF), prevalently affecting individuals over 60 years of age, has been mainly studied in young mouse models. The limited efficacy of current treatments underscores the need for animal models that better mimic an aged patient population. We addressed this by inducing pulmonary fibrosis in aged mice, using longitudinal micro-CT imaging as primary readout, with special attention to animal welfare.
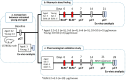
Methods: A double bleomycin dose was administered to 18-24 months-old male C57Bl/6j mice to induce pulmonary fibrosis. Bleomycin dosage was reduced to as low as 75% compared to that commonly administered to young (8-12 weeks-old) mice, resulting in long-term lung fibrosis without mortality, complying with animal welfare guidelines. After fibrosis induction, animals received Nintedanib once-daily for two weeks and longitudinally monitored by micro-CT, which provided structural and functional biomarkers, followed by post-mortem histological analysis as terminal endpoint.
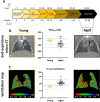
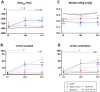
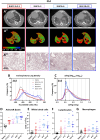
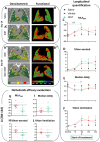
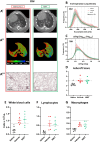
Results: Compared to young mice, aged animals displayed increased volume, reduced tissue density and function, and marked inflammation. This increased vulnerability imposed a bleomycin dosage reduction to the lowest tested level (2.5 µg/mouse), inducing a milder, yet persistent, fibrosis, while preserving animal welfare. Nintedanib treatment reduced fibrotic lesions and improved pulmonary function.
Conclusions: Our data identify a downsized bleomycin treatment that allows to achieve the best trade-off between fibrosis induction and animal welfare, a requirement for antifibrotic drug testing in aged lungs. Nintedanib displayed significant efficacy in this lower-severity disease model, suggesting potential patient stratification strategies. Lung pathology was quantitatively assessed by micro-CT, pointing to the value of longitudinal endpoints in clinical trials.
Keywords: Age; Bleomycin model; IPF; Lung fibrosis; Micro-computed tomography; Nintedanib.
© 2024. The Author(s).
Conflict of interest statement
FFS, AG, EF and GV are employees of Chiesi Farmaceutici S.p.A., that supported the research work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

