Airway epithelium damage in acute respiratory distress syndrome
- PMID: 39478566
- PMCID: PMC11523598
- DOI: 10.1186/s13054-024-05127-3
Airway epithelium damage in acute respiratory distress syndrome
Abstract
Background: The airway epithelium (AE) fulfils multiple functions to maintain pulmonary homeostasis, among which ensuring adequate barrier function, cell differentiation and polarization, and actively transporting immunoglobulin A (IgA), the predominant mucosal immunoglobulin in the airway lumen, via the polymeric immunoglobulin receptor (pIgR). Morphological changes of the airways have been reported in ARDS, while their detailed features, impact for mucosal immunity, and causative mechanisms remain unclear. Therefore, this study aimed to assess epithelial alterations in the distal airways of patients with ARDS.
Methods: We retrospectively analyzed lung tissue samples from ARDS patients and controls to investigate and quantify structural and functional changes in the small airways, using multiplex fluorescence immunostaining and computer-assisted quantification on whole tissue sections. Additionally, we measured markers of mucosal immunity, IgA and pIgR, alongside with other epithelial markers, in the serum and the broncho-alveolar lavage fluid (BALF) prospectively collected from ARDS patients and controls.
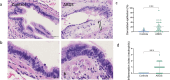
Results: Compared to controls, airways of ARDS were characterized by increased epithelial denudation (p = 0.0003) and diffuse epithelial infiltration by neutrophils (p = 0.0005). Quantitative evaluation of multiplex fluorescence immunostaining revealed a loss of ciliated cells (p = 0.0317) a trend towards decreased goblet cells (p = 0.056), and no change regarding cell progenitors (basal and club cells), indicating altered mucociliary differentiation. Increased epithelial permeability was also shown in ARDS with a significant decrease of tight (p < 0.0001) and adherens (p = 0.025) junctional proteins. Additionally, we observed a significant decrease of the expression of pIgR, (p < 0.0001), indicating impaired mucosal IgA immunity. Serum concentrations of secretory component (SC) and S-IgA were increased in ARDS (both p < 0.0001), along other lung-derived proteins (CC16, SP-D, sRAGE). However, their BALF concentrations remained unchanged, suggesting a spillover of airway and alveolar proteins through a damaged AE.
Conclusion: The airway epithelium from patients with ARDS exhibits multifaceted alterations leading to altered mucociliary differentiation, compromised defense functions and increased permeability with pneumoproteinemia.
Keywords: ARDS; Airway epithelium; Ciliated cells; Immunoglobulin A; Junctional proteins; Mucosal immunity; Pneumoproteinemia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

