Characterization of gut microbiota dynamics in an Alzheimer's disease mouse model through clade-specific marker-based analysis of shotgun metagenomic data
- PMID: 39478626
- PMCID: PMC11524029
- DOI: 10.1186/s13062-024-00541-7
Characterization of gut microbiota dynamics in an Alzheimer's disease mouse model through clade-specific marker-based analysis of shotgun metagenomic data
Abstract
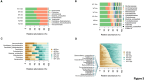
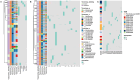
Alzheimer's disease (AD) is a complex neurodegenerative disorder significantly impairing cognitive faculties, memory, and physical abilities. To characterize the modulation of the gut microbiota in an in vivo AD model, we performed shotgun metagenomics sequencing on 3xTgAD mice at key time points (i.e., 2, 6, and 12 months) of AD progression. Fecal samples from both 3xTgAD and wild-type mice were collected, DNA extracted, and sequenced. Quantitative taxon abundance assessment using MetaPhlAn 4 ensured precise microbial community representation. The analysis focused on species-level genome bins (SGBs) including both known and unknown SGBs (kSGBs and uSGBs, respectively) and also comprised higher taxonomic categories such as family-level genome bins (FGBs), class-level genome bins (CGBs), and order-level genome bins (OGBs). Our bioinformatic results pinpointed the presence of extensive gut microbial diversity in AD mice and showed that the largest proportion of AD- and aging-associated microbiome changes in 3xTgAD mice concern SGBs that belong to the Bacteroidota and Firmicutes phyla, along with a large set of uncharacterized SGBs. Our findings emphasize the need for further advanced bioinformatic studies for accurate classification and functional analysis of these elusive microbial species in relation to their potential bridging role in the gut-brain axis and AD pathogenesis.
Keywords: 3xTgAD mice; Alzheimer’s disease; Gut microbiota; MetaPhlAn 4; Shotgun metagenomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Vitale I, Pietrocola F, Guilbaud E, Aaronson SA, Abrams JM, Adam D, Agostini M, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Aqeilan RI, Arama E, Baehrecke EH, Balachandran S, Bano D, Barlev NA, Bartek J, Bazan NG, Becker C, Bernassola F, Bertrand MJM, Bianchi ME, Blagosklonny MV, Blander JM, Blandino G, Blomgren K, Borner C, Bortner CD, Bove P, Boya P, Brenner C, Broz P, Brunner T, Damgaard RB, Calin GA, Campanella M, Candi E, Carbone M, Carmona-Gutierrez D, Cecconi F, Chan FK, Chen GQ, Chen Q, Chen YH, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Ciliberto G, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Daugaard M, Dawson TM, Dawson VL, De Maria R, De Strooper B, Debatin KM, Deberardinis RJ, Degterev A, Del Sal G, Deshmukh M, Di Virgilio F, Diederich M, Dixon SJ, Dynlacht BD, El-Deiry WS, Elrod JW, Engeland K, Fimia GM, Galassi C, Ganini C, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Gerlic M, Ghosh S, Green DR, Greene LA, Gronemeyer H, Häcker G, Hajnóczky G, Hardwick JM, Haupt Y, He S, Heery DM, Hengartner MO, Hetz C, Hildeman DA, Ichijo H, Inoue S, Jäättelä M, Janic A, Joseph B, Jost PJ, Kanneganti TD, Karin M, Kashkar H, Kaufmann T, Kelly GL, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Kluck R, Krysko DV, Kulms D, Kumar S, Lavandero S, Lavrik IN, Lemasters JJ, Liccardi G, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Luedde T, MacFarlane M, Madeo F, Malorni W, Manic G, Mantovani R, Marchi S, Marine JC, Martin SJ, Martinou JC, Mastroberardino PG, Medema JP, Mehlen P, Meier P, Melino G, Melino S, Miao EA, Moll UM, Muñoz-Pinedo C, Murphy DJ, Niklison-Chirou MV, Novelli F, Núñez G, Oberst A, Ofengeim D, Opferman JT, Oren M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pentimalli F, Pereira DM, Pervaiz S, Peter ME, Pinton P, Porta G, Prehn JHM, Puthalakath H, Rabinovich GA, Rajalingam K, Ravichandran KS, Rehm M, Ricci JE, Rizzuto R, Robinson N, Rodrigues CMP, Rotblat B, Rothlin CV, Rubinsztein DC, Rudel T, Rufini A, Ryan KM, Sarosiek KA, Sawa A, Sayan E, Schroder K, Scorrano L, Sesti F, Shao F, Shi Y, Sica GS, Silke J, Simon HU, Sistigu A, Stephanou A, Stockwell BR, Strapazzon F, Strasser A, Sun L, Sun E, Sun Q, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Troy CM, Turk B, Urbano N, Vandenabeele P, Vanden Berghe T, Vander Heiden MG, Vanderluit JL, Verkhratsky A, Villunger A, von Karstedt S, Voss AK, Vousden KH, Vucic D, Vuri D, Wagner EF, Walczak H, Wallach D, Wang R, Wang Y, Weber A, Wood W, Yamazaki T, Yang HT, Zakeri Z, Zawacka-Pankau JE, Zhang L, Zhang H, Zhivotovsky B, Zhou W, Piacentini M, Kroemer G, Galluzzi L. Cell Death Differ. 2023;30:1097–154. Apoptotic cell death in disease-Current understanding of the NCCD 2023. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

