A Continuous and Noninvasive Method to Estimate Pao2/Fio2 Ratio
- PMID: 39478812
- PMCID: PMC11519398
- DOI: 10.1097/CCE.0000000000001174
A Continuous and Noninvasive Method to Estimate Pao2/Fio2 Ratio
Abstract
Objectives: To validate a method for continuously estimating the Pao2/Fio2 (PF) ratio in all critically ill pediatric patients using only standard continuous data monitoring.
Design: Retrospective study on a high temporal resolution database.
Setting: PICU in Montreal, QC, Canada.
Patients/subjects: We included any patients admitted from May 2015 to May 2023 who had an arterial blood gas (ABG) with concurrent continuous pulsed oximetry saturation (Spo2) values. We used our previously validated mathematical model to determine the magnitude of hypoxemia by computing the estimated ePao2/Fio2 (ePF) ratio and comparing it to the Spo2/Fio2 (SF), using PF ratio as the reference standard.
Interventions: None.
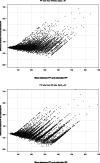
Measurements and main results: We analyzed a total of 20,828 ABGs. When Spo2 was below or equal to 97%, the ePF ratio showed a significantly better hypoxemia classification (none, light/moderate, or severe) than the SF ratio (0.80 vs. 0.72; p < 0.001), a lower fixed bias (16.26 vs. -35.24; p < 0.001), a lower mean absolute error (37.92 vs. 63.93; p < 0.001) and a lower proportional bias (slope of 1.01 vs. 0.81; p < 0.001). ePF ratio has also a better limits of agreement difference from Bland-Altman plot (248.10 vs. 292.45; p < 0.001) and coefficient of determination (0.68 vs. 0.59; p < 0.001). When Spo2 was above 97%, the ePF ratio had better classification with Kappa (0.53 vs. 0.43; p < 0.001) and lower fixed bias (-0.63 vs. 65.68; p < 0.001).
Conclusions: The PF ratio based on ePF allows for a continuous estimation of hypoxemia severity with a better performance than the SF ratio.
Keywords: automatic data processing; clinical decision support systems; critical care; hypoxemia.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Santschi M, Jouvet P, Leclerc F, et al. : Acute lung injury in children: Therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010; 11:681–689 - PubMed
-
- Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 - PubMed
-
- Khemani RG, Smith L, Lopez-Fernandez YM, et al. ; Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) Investigators: Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): An international observational study. Lancet Respir Med 2019; 7:115–128 - PMC - PubMed
-
- Eytan D, Mazwi ML, Goodwin AJ, et al. : Revisiting oxygen dissociation curves and bedside measured arterial saturation in critically ill children. Intensive Care Med 2019; 45:1832–1834 - PubMed
-
- Khemani RG, Thomas NJ, Venkatachalam V, et al. ; Pediatric Acute Lung Injury and Sepsis Network Investigators (PALISI): Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 2012; 40:1309–1316 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

