Back pain exercise therapy remodels human epigenetic profiles in buccal and human peripheral blood mononuclear cells: an exploratory study in young male participants
- PMID: 39478832
- PMCID: PMC11521823
- DOI: 10.3389/fspor.2024.1393067
Back pain exercise therapy remodels human epigenetic profiles in buccal and human peripheral blood mononuclear cells: an exploratory study in young male participants
Abstract
Background: With its high and increasing lifetime prevalence, back pain represents a contemporary challenge for patients and healthcare providers. Monitored exercise therapy is a commonly prescribed treatment to relieve pain and functional limitations. However, the benefits of exercise are often gradual, subtle, and evaluated by subjective self-reported scores. Back pain pathogenesis is interlinked with epigenetically mediated processes that modify gene expression without altering the DNA sequence. Therefore, we hypothesize that therapy effects can be objectively evaluated by measurable epigenetic histone posttranslational modifications and proteome expression. Because epigenetic modifications are dynamic and responsive to environmental exposure, lifestyle choices-such as physical activity-can alter epigenetic profiles, subsequent gene expression, and health traits. Instead of invasive sampling (e.g., muscle biopsy), we collect easily accessible buccal swabs and plasma. The plasma proteome provides a systemic understanding of a person's current health state and is an ideal snapshot of downstream, epigenetically regulated, changes upon therapy. This study investigates how molecular profiles evolve in response to standardized sport therapy and non-controlled lifestyle choices.
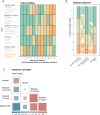
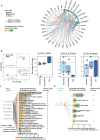
Results: We report that the therapy improves agility, attenuates back pain, and triggers healthier habits. We find that a subset of participants' histone methylation and acetylation profiles cluster samples according to their therapy status, before or after therapy. Integrating epigenetic reprogramming of both buccal cells and peripheral blood mononuclear cells (PBMCs) reveals that these concomitant changes are concordant with higher levels of self-rated back pain improvement and agility gain. Additionally, epigenetic changes correlate with changes in immune response plasma factors, reflecting their comparable ability to rate therapy effects at the molecular level. We also performed an exploratory analysis to confirm the usability of molecular profiles in (1) mapping lifestyle choices and (2) evaluating the distance of a given participant to an optimal health state.
Conclusion: This pre-post cohort study highlights the potential of integrated molecular profiles to score therapy efficiency. Our findings reflect the complex interplay of an individual's background and lifestyle upon therapeutic exposure. Future studies are needed to provide mechanistic insights into back pain pathogenesis and lifestyle-based epigenetic reprogramming upon sport therapy intervention to maintain therapeutic effects in the long run.
Keywords: back pain; biomarker; data integration; epigenetics; histone modifications; lifestyle exposome; plasma proteome; sport therapy.
© 2024 Burny, Potočnjak, Hestermann, Gartemann, Hollmann, Schifferdecker-Hoch, Markanovic, Di Sanzo, Günsel, Solis-Mezarino and Voelker-Albert.
Conflict of interest statement
CB, MP, NM, SS, MG, VS-M, and MV-A were employees of EpiQMAx GmbH. MP, SS, and MV-A are employees of MOLEQLAR Analytics GmbH. AH, SG, MH, and FS are employees of FPZ GmbH.
Figures





References
-
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43(7):1334–59. 10.1249/MSS.0B013E318213FEFB - DOI - PubMed
LinkOut - more resources
Full Text Sources

