Energy exchange between Nd3+ and Er3+ centers within molecular complexes
- PMID: 39479154
- PMCID: PMC11515939
- DOI: 10.1039/d4sc03994g
Energy exchange between Nd3+ and Er3+ centers within molecular complexes
Abstract
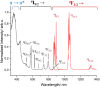
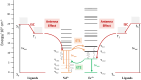
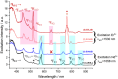
Developing controlled and reproducible molecular assemblies incorporating lanthanide centers is a crucial step for driving forward up- and down-conversion processes. This challenge calls for the development of strategies to facilitate the efficient in situ segregation of different Ln metal ions into distinct positions within the molecule. The unique family of pure [LnLn'Ln] heterometallic coordination compounds previously developed by us represents an ideal platform for studying the desired Ln-to-Ln' energy transfer (ET). In this context, we report here the new pure one-step synthetically produced [ErNdEr] (3) complex, which allows for the first time at the molecular level to study the mechanisms behind Nd-to-Er energy transfer. To further assess the photophysical properties of this complex, the analogous [LuNdLu] (1) and [ErLaEr] (2) complexes have also been prepared and photophysically studied. Efficient sensitization via the two β-diketones employed as main ligands was probed for both Nd3+ and Er3+ ions, resulting in highly resolved emission spectra and sufficiently long excited state lifetimes, which allowed further assessment of the Ln-to-Ln' ET. This intermetallic transfer was first detected by comparing the emission spectra of iso-absorbant solutions and demonstrated by comparing the lifetime values with or without the lanthanide quencher (Er3+), as well as with a deep analysis of the excitation spectrum of the three complexes. Thus, a very unique phenomenon was discovered, consisting of a mutual Nd-to-Er and Er-to-Nd ET with no net increase of brightness by any metal; while Nd3+ transfers the energy received from the antenna to Er3+, the sensitization of the latter results in back-transfer to Nd3+ into a non-emissive, thus silent, state.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
LinkOut - more resources
Full Text Sources

