Adaptive photoluminescence through a bioinspired antioxidative mechanism
- PMID: 39479160
- PMCID: PMC11515932
- DOI: 10.1039/d4sc06096b
Adaptive photoluminescence through a bioinspired antioxidative mechanism
Abstract
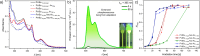
Transition metal complexes are archetypal luminescent probes that are widely used for various applications ranging from optoelectronics to biomedicine. However, they face significant challenges such as photobleaching and photooxidative stress, which limit their performance. Herein, we introduce a photosystem-inspired concept based on the use of a vitamin (ascorbic acid, Asc-Ac) to adaptively suppress photobleaching of molecular luminophores. As a proof-of-concept compound, we have selected a new bis-cyclometalated Pt(II) complex (Pt-tBu) and investigated its adaptive photoluminescence resulting from singlet dioxygen (1O2) photoproduction in the presence of Asc-Ac. Interestingly, the excited state quenching and subsequent photobleaching of Pt-tBu in aerated solutions is suppressed by addition of Asc-Ac, which scavenges the 1O2 photosensitized by Pt-tBu upon irradiation and results in an adaptive oxygen depletion with enhancement of luminescence. The adaptation is resilient for successive irradiation cycles with oxygen replenishment, until peroxidation overshooting leads to the degradation of Pt-tBu by formation of a dark Pt(iv) species. The complexity-related adaptation with initial overperformance (luminescence boost) relies on the external energy input and cascaded feedback loops, thus biomimicking inflammation, as the repeated exposure to a stressor leads to a final breakdown. Our antioxidative protection mechanism against photobleaching can be successfully extended to multiple coordination compounds (e.g., Ir(iii), Ru(ii) and Re(i) complexes), thus demonstrating its generality. Our findings broaden the scope of molecular adaptation and pave the way for enhancing the stability of molecular luminophores for multiple applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

