Metal ion cofactors modulate integral enzyme activity by varying differential membrane curvature stress
- PMID: 39479198
- PMCID: PMC11514723
- DOI: 10.1039/d4lf00309h
Metal ion cofactors modulate integral enzyme activity by varying differential membrane curvature stress
Abstract
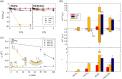
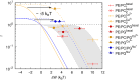
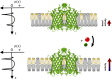
Metal ions are well-known cofactors of protein function and stability. In the case of the integral membrane enzyme OmpLA (outer membrane phospholipase A) the active dimer is stabilized by calcium ions. We studied the lipid hydrolysis kinetics of OmpLA in charge-neutral and charged membranes with symmetric or asymmetric transbilayer lipid distributions. In charge-neutral membranes, OmpLA was more active in symmetric bilayers due to the lower differential curvature stress between membrane leaflets. Strikingly, this behavior was completely reversed in charged bilayers. Measurements revealed intrinsic molecular shape changes in the charged lipids upon addition of calcium. This effectively reduces the differential curvature stress in charged asymmetric membranes leading to increased protein activity. This conclusion is further supported by similar effects observed upon the addition of sodium ions, which also alter the shape of the lipids, but do not specifically interact with the protein. Additional lipid-protein interactions likely contribute to this phenomenon. Our findings demonstrate that ion cofactors not only interact directly with membrane proteins but also modulate protein activity indirectly by altering the effective molecular shape of charged lipid species.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
LinkOut - more resources
Full Text Sources
