New specimens of Bunaia woodwardi Clarke, 1919 (Euchelicerata): a new member of Offacolidae providing insight supporting the Arachnomorpha
- PMID: 39479250
- PMCID: PMC11524597
- DOI: 10.1098/rsos.240499
New specimens of Bunaia woodwardi Clarke, 1919 (Euchelicerata): a new member of Offacolidae providing insight supporting the Arachnomorpha
Abstract
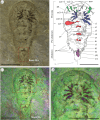
The rapid early diversification of arthropods has made understanding internal relationships within the group fiendish. Particularly unresolved is the origin of Euchelicerata, a clade consisting of the Prosomapoda (comprising the extant Xiphosura and Arachnida and the extinct Chasmataspidida, Eurypterida and synziphosurines) and the extinct Offacolidae. Here we describe new material of the Silurian 'synziphosurine' Bunaia woodwardi Clarke, 1919 that reveals previously unknown features of its ventral anatomy: a pair of elongated chelicerae in the prosoma, followed posteriorly by five pairs of biramous appendages, a first pre-abdomen somite bearing a pair of paddle-like uniramous appendages (exopods) and a ventral pretelsonic process. Phylogenetic analyses retrieve B. woodwardi as a member of Offacolidae closely related to Setapedites abundantis from the Early Ordovician Fezouata Biota. An anatomical comparison of the pretelsonic process of B. woodwardi, also present in Setapedites, with the posterior trunk morphologies of other Offacolidae, Habeliida and Vicissicaudata, suggests a possible homologous appendicular origin. This proposed apomorphic character supports a monophyletic Arachnomorpha, formed of Vicissicaudata, Habeliida and Euchelicerata. The establishment of this new homology could help to clarify the highly enigmatic phylogeny at the base of the euchelicerates as well as the sequence of character acquisition during their early evolution.
Keywords: Arachnomorpha; Artiopoda; Euchelicerata; Offacolidae; Vicissicaudata; synziphosurine.
© 2024 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures








Similar articles
-
Lower Ordovician synziphosurine reveals early euchelicerate diversity and evolution.Nat Commun. 2024 May 7;15(1):3808. doi: 10.1038/s41467-024-48013-w. Nat Commun. 2024. PMID: 38714651 Free PMC article.
-
The appendicular morphology of Sinoburius lunaris and the evolution of the artiopodan clade Xandarellida (Euarthropoda, early Cambrian) from South China.BMC Evol Biol. 2019 Aug 6;19(1):165. doi: 10.1186/s12862-019-1491-3. BMC Evol Biol. 2019. PMID: 31387545 Free PMC article.
-
Geological history and phylogeny of Chelicerata.Arthropod Struct Dev. 2010 Mar-May;39(2-3):124-42. doi: 10.1016/j.asd.2010.01.003. Epub 2010 Mar 20. Arthropod Struct Dev. 2010. PMID: 20093195 Review.
-
The Vicissicaudata revisited - insights from a new aglaspidid arthropod with caudal appendages from the Furongian of China.Sci Rep. 2017 Sep 11;7(1):11117. doi: 10.1038/s41598-017-11610-5. Sci Rep. 2017. PMID: 28894246 Free PMC article.
-
Segmentation and tagmosis in Chelicerata.Arthropod Struct Dev. 2017 May;46(3):395-418. doi: 10.1016/j.asd.2016.05.002. Epub 2016 Jun 21. Arthropod Struct Dev. 2017. PMID: 27240897 Review.
Cited by
-
The first Silurian horseshoe crab reveals details of the xiphosuran ground plan.Proc Biol Sci. 2025 Jun;292(2049):20250874. doi: 10.1098/rspb.2025.0874. Epub 2025 Jun 18. Proc Biol Sci. 2025. PMID: 40527460 Free PMC article.
References
-
- Giribet G. 2018. Current views on chelicerate phylogeny—a tribute to Peter Weygoldt. Zool. Anz. 273, 7–13. (10.1016/j.jcz.2018.01.004) - DOI
Associated data
LinkOut - more resources
Full Text Sources

