Preventing Cardiac Damage in Patients Treated for Breast Cancer and Lymphoma: The PROACT Clinical Trial
- PMID: 39479329
- PMCID: PMC11520224
- DOI: 10.1016/j.jaccao.2024.07.010
Preventing Cardiac Damage in Patients Treated for Breast Cancer and Lymphoma: The PROACT Clinical Trial
Abstract
Background: Cardiotoxicity is a concern for cancer survivors undergoing anthracycline chemotherapy. Enalapril has been explored for its potential to mitigate cardiotoxicity in cancer patients. The dose-dependent cardiotoxicity effects of anthracyclines can be detected early through the biomarker cardiac troponin.
Objectives: The PROACT (Preventing Cardiac Damage in Patients Treated for Breast Cancer and Lymphoma) clinical trial assessed the effectiveness of enalapril in preventing cardiotoxicity, manifesting as myocardial injury and cardiac function impairment, in patients undergoing high-dose anthracycline-based chemotherapy for breast cancer or non-Hodgkin lymphoma.
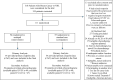
Methods: This prospective, multicenter, open-label, randomized controlled trial employed a superiority design with observer-blinded endpoints. A total of 111 participants, scheduled for 6 cycles of chemotherapy with a planned dose of ≥300 mg/m2 doxorubicin equivalents, were randomized to receive either enalapril (titrated up to 20 mg daily) or standard care without enalapril.
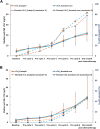
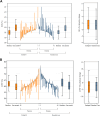
Results: Myocardial injury, indicated by cardiac troponin T (≥14 ng/L), during and 1 month after chemotherapy, was observed in 42 (77.8%) of 54 patients in the enalapril group vs 45 (83.3%) of 54 patients in the standard care group (OR: 0.65; 95% CI: 0.23-1.78). Injury detected by cardiac troponin I (>26.2 ng/L) occurred in 25 (47.2%) of 53 patients on enalapril compared with 24 (45.3%) of 53 in standard care (OR: 1.10; 95% CI: 0.50-2.38). A relative decline of more than 15% from baseline in left ventricular global longitudinal strain was observed in 10 (21.3%) of 47 patients on enalapril and 9 (21.9%) of 41 in standard care (OR: 0.95; 95% CI: 0.33-2.74). An absolute decline of >10% to <50% in left ventricular ejection fraction was seen in 2 (4.1%) of 49 patients on enalapril vs none in patients in standard care.
Conclusions: Adding enalapril to standard care during chemotherapy did not prevent cardiotoxicity in patients receiving high-dose anthracycline-based chemotherapy. (PROACT: Can we prevent Chemotherapy-related Heart Damage in Patients With Breast Cancer and Lymphoma?; NCT03265574).
Keywords: anthracycline; biomarkers; breast cancer; echocardiography; lymphoma; prevention.
© 2024 The Authors.
Conflict of interest statement
This work was supported by the National Institute for Health and Care Research (PB-PG-0815-20061). Dr Gilbert was supported by a grant from JGW Patterson Foundation. Dr Mills was supported by a Chair Award (CH/F/21/90010), Programme Grant (RG/20/10/34966), and Research Excellence Award (RE/24/130012) from the British Heart Foundation. Dr Austin has received speaker fees from Philips Volcano, AstraZeneca, and Pfizer; and research grants awarded to Newcastle University from TA Sciences, Kancera, and AstraZeneca. Dr Maier has received research grants awarded to Newcastle University from TA Sciences, Kancera, and AstraZeneca. Dr Maddox has received funding to attend meetings from Novartis and AbbVie. Dr Mills has received research grants awarded to the University of Edinburgh from Abbott Diagnostics, Siemens Healthineers, and Roche Diagnostics, outside the submitted work; and honoraria from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics, LumiraDx, and Psyros Diagnostics. Dr Kasim was an employee of Durham University during his involvement in the PROACT trial, and is now an employee of GlaxoSmithKline. Dr Plummer has received speaker fees or travel expenses from Amgen, BeiGene, Calgene, Incyte, Ipsen, Novartis, and Servier. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–1983. doi: 10.1002/ejhf.2017. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

