Biomineralized in situ catalytic nanoreactor integrated microneedle patch for on demand immunomodulator supply to combat psoriasis
- PMID: 39479439
- PMCID: PMC11519795
- DOI: 10.7150/thno.101845
Biomineralized in situ catalytic nanoreactor integrated microneedle patch for on demand immunomodulator supply to combat psoriasis
Abstract
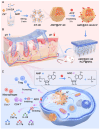
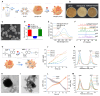
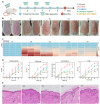
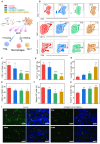
The endogenous immunomodulator adenosine (ADO) was expected to be potentialized as an efficacious mediator to combat psoriasis. However, its efficacy is severely hindered by its poor metabolic stability and insufficient accumulation at the dermatological lesions. Methods: In this study, a biomineralized in situ catalytic nanoreactor was delicately customized by encapsulating ADO precursor (adenosine monophosphate, AMP) within the internal porous skeleton of zeolitic imidazolate framework-90, followed by the biomineralization of the AMP catabolic enzyme on the outer layer. The nanocrystals were then incorporated into a dissolving microneedles patch, which was designed to deliver drugs with precision into the cutaneous lesion and enhance the efficacy of psoriasis treatment. Results: Upon penetration into the skin, the nanoreactors were released and underwent a gradual collapse of their structure, releasing AMP when exposed to the acidic microenvironment. Meanwhile, the acidic pH could trigger an in situ catalytic reaction to continuously produce ADO. This system yielded remarkable results in a psoriasis-like mouse model. The mechanism study demonstrated that this system could substantially reshape the inflammatory ecosystem by inhibiting the keratinocyte hyperplasia, reducing inflammatory cytokine expression, and regulating the infiltration of immune cells. Conclusion: The in situ catalytic nanoreactor integrated microneedle patch is a promising modular platform for co-delivery of prodrugs and their catabolic enzymes, offering a potential solution for various diseases.
Keywords: adenosine; immunoregulation; in situ catalytic nanoreactor; microneedles; psoriasis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Smart core-shell microneedles for psoriasis therapy: In situ self-assembly of calcium ion-coordinated dexamethasone hydrogel.J Control Release. 2025 Mar 10;379:786-796. doi: 10.1016/j.jconrel.2025.01.037. Epub 2025 Jan 25. J Control Release. 2025. PMID: 39828209
-
Screening and preparation of curcumin nano-formulations combined with dissolving microneedles on the application in the effective treatment of psoriasis.Int J Pharm. 2025 Apr 30;675:125528. doi: 10.1016/j.ijpharm.2025.125528. Epub 2025 Mar 27. Int J Pharm. 2025. PMID: 40157563
-
Calcipotriol Nanosuspension-Loaded Trilayer Dissolving Microneedle Patches for the Treatment of Psoriasis: In Vitro Delivery and In Vivo Antipsoriatic Activity Studies.Mol Pharm. 2024 Jun 3;21(6):2813-2827. doi: 10.1021/acs.molpharmaceut.3c01223. Epub 2024 May 16. Mol Pharm. 2024. PMID: 38752564 Free PMC article.
-
Dissolving Microneedle Patches for Dermal Vaccination.Pharm Res. 2017 Nov;34(11):2223-2240. doi: 10.1007/s11095-017-2223-2. Epub 2017 Jul 17. Pharm Res. 2017. PMID: 28718050 Free PMC article. Review.
-
Polyphenol Microneedles for Dermatological Therapy.Macromol Biosci. 2025 Jun;25(6):e2400607. doi: 10.1002/mabi.202400607. Epub 2025 Feb 28. Macromol Biosci. 2025. PMID: 40017440 Review.
References
-
- Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983–94. - PubMed
-
- Zhao P, Li Z, Ling Z, Zheng Y, Chang H. Efficient loading and sustained delivery of methotrexate using a tip-swellable microneedle array patch for psoriasis treatment. ACS Biomater Sci Eng. 2024;10:921–31. - PubMed
-
- Sewerin P, Brinks R, Schneider M, Haase I, Vordenbaumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2019;78:286–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

