Macrophage membrane‒biomimetic nanoparticles target inflammatory microenvironment for epilepsy treatment
- PMID: 39479447
- PMCID: PMC11519803
- DOI: 10.7150/thno.99260
Macrophage membrane‒biomimetic nanoparticles target inflammatory microenvironment for epilepsy treatment
Abstract
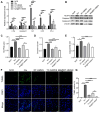
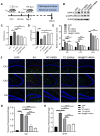
Rationale: The clinical treatment of epilepsy is faced with challenges. On the one hand, the effectiveness of existing antiepileptic drugs (AEDs) is limited by the blood‒brain barrier (BBB); on the other hand, changes in the inflammatory microenvironment during epileptogenesis are often neglected. Methods: The death-associated protein kinase 1 inhibitor TC-DAPK6 and the fluorescent probe rhodamine B were encapsulated in hollow mesoporous silica nanocarriers (HMSNs), which were then coated with a macrophage membrane to prepare macrophage membrane-biomimetic nanoparticles, namely, MA@RT-HMSNs. In vitro biotoxicity, cellular uptake, BBB permeability and inflammatory targeting ability were evaluated in cells. The effects of MA@RT-HMSN treatment were explored by immunohistochemistry, TUNEL assay, Western blot analysis, quantitative real-time polymerase chain reaction, electroencephalogram recording and behavioural tests in kainic acid-induced acute and chronic epilepsy model mice. Results: MA@RT-HMSNs showed excellent biocompatibility both in vitro and in vivo. MA@RT-HMSNs successfully crossed the BBB and exhibited increased efficacy in targeted delivery of TC-DAPK6 to inflammatory lesions in epileptic foci. Macrophage membrane coating conferred MA@RT-HMSNs with higher stability, greater cellular uptake, and enhanced TC-DAPK6 bioavailability. Furthermore, MA@RT-HMSNs exerted beneficial therapeutic effects on acute and chronic epilepsy models by alleviating microenvironment inflammation, preventing neuronal death, and inhibiting neuronal excitability and gliosis. Conclusions: MA@RT-HMSNs target inflammatory foci to inhibit death-related protein kinase 1 and exert antiepileptic effects. This study provides a promising biomimetic nanodelivery system for targeted epilepsy therapy.
Keywords: death-related protein kinase 1; drug delivery; epilepsy; inflammation; macrophage membrane biomimetic nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

