Disease Burden and Access to Biologic Therapy in Patients with Severe Asthma, 2017-2022: An Analysis of the International Severe Asthma Registry
- PMID: 39479509
- PMCID: PMC11522015
- DOI: 10.2147/JAA.S468068
Disease Burden and Access to Biologic Therapy in Patients with Severe Asthma, 2017-2022: An Analysis of the International Severe Asthma Registry
Abstract
Introduction: Patients with severe asthma may be prescribed biologic therapies to improve disease control. The EVEREST study aimed to characterize the global disease burden of patients with severe asthma without access to biologics and those who have access but do not receive biologics, as well as the remaining unmet need despite use of these therapies.
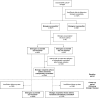
Methods: This was a historical cohort study of patients with severe asthma (aged ≥18 years) in the International Severe Asthma Registry receiving Global Initiative for Asthma (GINA) 2018 step 5 treatment, or with uncontrolled disease at GINA step 4. Prospective data on patient clinical characteristics, healthcare resource utilization, and medication use over a 12-month period between December 2017 and May 2022 were assessed for the following five groups: biologics accessible (omalizumab, mepolizumab, reslizumab, benralizumab, or dupilumab); biologics inaccessible; biologics accessible but not received; biologics accessible and received; and biologic recipients whose asthma remained suboptimally controlled.
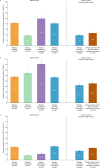
Results: Overall, 9587 patients from 21 countries were included. Among patients in the biologics accessible (n=5073), biologics inaccessible (n=3041), and biologics accessible but not received (n=382) groups, 41.4%, 18.7%, and 49.6% experienced at least two exacerbations, 11.5%, 10.5%, and 6.2% required at least one hospitalization, 47.9%, 54.6%, and 71.2% had uncontrolled asthma, and 23.9%, 8.6%, and 11.0% received long-term oral corticosteroids (LTOCS), respectively. Following biologic therapy, among patients who received biologics overall (n=2666) and among those whose asthma remained suboptimally controlled (n=1780), 19.1% and 23.0% experienced at least two exacerbations, 2.7% and 2.9% required at least one hospitalization, and 16.7% and 22.0% received LTOCS, respectively.
Conclusion: There is a substantial disease burden in both patients without access to biologics and those with access who do not receive these therapies, although specific outcomes may vary between these groups. There also remains a high unmet need among biologic recipients, many of whom have a suboptimal response to treatment.
Keywords: biologic; disease burden; healthcare resource utilization; severe asthma.
© 2024 Le et al.
Conflict of interest statement
Tham T. Le, Clement Erhard, Bill Cook, Anna Quinton, Neil Martin, and Trung N. Tran are employees of AstraZeneca and may own stock or stock options in AstraZeneca. David B Price has participated in advisory boards with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermo Fisher, and Viatris; has consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GSK, Medscape, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance, and Viatris; has received grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, the UK National Health Service, and Viatris; has received payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GSK, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, and Viatris; has received payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Circassia, Mundipharma, Novartis, Teva Pharmaceuticals, and Thermo Fisher; has received funding for patient enrollment or completion of research from Novartis; has stock or stock options with AKL Research and Development Ltd; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); owns 5% shareholding in Timestamp; is a peer reviewer for Health Technology Assessment and the grant committees of the UK Efficacy and Mechanism Evaluation Programme; and was an expert witness for GSK. Rohit Katial is a former employee of AstraZeneca and has been an advisory board participant and speaker for GSK and Sanofi/Regeneron. Luis Perez-de-Llano has received grants from AstraZeneca, Chiesi, and Teva Pharmaceuticals; personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, FAES, GEBRO, GSK, Mundipharma, Novartis, Sanofi, and Teva Pharmaceuticals; and nonfinancial support from Boehringer Ingelheim, Esteve, GSK, Menarini, Mundipharma, Novartis, and Teva Pharmaceuticals. Alan Altraja has received lecture fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GSK, MSD, Norameda, Novartis, Orion, Sanofi Regeneron, and Zentiva; sponsorships from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GSK, MSD, Norameda, Novartis, and Sanofi Regeneron; and has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, CSL Behring, GSK, Novartis, Sanofi Regeneron, and Teva Pharmaceuticals. Celine Bergeron has participated in advisory boards for GSK, Sanofi-Regeneron, AstraZeneca, Amgen, Takeda, and Valeo Pharma; has received honoraria for presentations for AstraZeneca, Amgen, Grifols, GSK, Sanofi-Regeneron, and Valeo Pharma; and her institution has received grants from AstraZeneca, Biohaven, GSK, OPRI/ISAR, Novartis, Sanofi-Regeneron and Teva. Arnaud Bourdin has received industry-sponsored grants from AstraZeneca, Boehringer Ingelheim, Cephalon/Teva, GSK, Novartis, and Sanofi-Regeneron; and been a consultant for Actelion, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, MedinCell, Merck, Novartis, Regeneron-Sanofi, and Roche. Mariko Siyue Koh has received grants from AstraZeneca; and honoraria for lectures and advisory board meetings paid to her hospital (Singapore General Hospital) from GSK, AstraZeneca, Boehringer Ingelheim, Novartis, Roche, and Sanofi. Lauri Lehtimäki has received personal fees from ALK, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis, Orion Pharma, and Sanofi. Nikolaos G Papadopoulos has been a speaker and/or advisory board member for Abbott, AbbVie, ALK, Asit Biotech, AstraZeneca, Biomay, Boehringer Ingelheim, GSK, HAL, Faes Farma, Medscape, Menarini, MSD, Mylan, Novartis, Nutricia, OM Pharma, Regeneron, Sanofi, Takeda, and Viatris. Paul Pfeffer has attended advisory boards for AstraZeneca, GSK, and Sanofi; has given lectures at meetings supported by AstraZeneca and GSK; has taken part in clinical trials sponsored by AstraZeneca, GSK, Novartis, and Sanofi, for which his institution received remuneration; has received speaker fee from Chiesi for an educational webinar and has a current research grant funded by GSK. Chin Kook Rhee received consulting/lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, GSK, MSD, Mundipharma, Novartis, Sanofi, Takeda, and Teva. Victoria Carter is an employee of Optimum Patient Care, a co-funder of ISAR. The authors report no other conflicts of interest in this work.
Figures
References
-
- World Health Organization. Fact sheets: asthma. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed February 22, 2024.
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2023. Available from: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-2.... Accessed February 22, 2024.
LinkOut - more resources
Full Text Sources

