Comparative efficacy of lisocabtagene maraleucel in the PILOT study versus second-line chemotherapy regimens in the real world
- PMID: 39479862
- PMCID: PMC11873703
- DOI: 10.3324/haematol.2024.285828
Comparative efficacy of lisocabtagene maraleucel in the PILOT study versus second-line chemotherapy regimens in the real world
Abstract
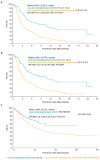
This study assessed the comparative efficacy of lisocabtagene maraleucel (liso-cel) in the open-label, phase II PILOT study (clinicaltrials.gov NCT03483103) versus conventional second-line (2L) chemotherapy regimens in the real world administered to patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who were not intended for hematopoietic stem cell transplantation (HSCT). The liso-cel-treated cohort (N=61) was based on patients who received liso-cel in the PILOT study. The conventional chemotherapy cohort included patients who met PILOT eligibility criteria and received conventional 2L chemotherapy in the real-world clinical setting (N=273). After using the trimmed stabilized inverse probability of treatment weighting method to balance cohorts according to baseline characteristics, there were statistically significant differences in all tested measures of efficacy. Compared with real-world conventional chemotherapy regimens, liso-cel demonstrated higher overall response rates (79.6% with liso-cel vs. 50.5% with conventional chemotherapy; relative risk [RR]: 1.6; P<0.0001) and complete response rates (53.1% vs. 24.0%; RR: 2.2; P<0.0001), longer median duration of response (12.1 vs. 4.3 months; hazard ratio [HR: 0.40; P=0.0001), longer median event-free survival (7.0 vs. 2.8 months; HR: 0.43; P<0.0001), longer median progression-free survival (7.0 vs. 2.9 months; HR: 0.46; P<0.0001), and longer median overall survival (not reached vs. 12.6 months; HR: 0.58; P=0.0256). Results from analyses applying various additional statistical approaches consistently favored outcomes with liso-cel over real-world conventional chemotherapy regimens. These results reinforce the efficacy of liso-cel as 2L therapy for patients with R/R LBCL who are not intended for HSCT.
Figures

References
-
- Locke FL, Miklos DB, Jacobson CA, et al. .; for All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. - PubMed
-
- Crump M, Kuruvilla J, Couban S, et al. . Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490-3496. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

