Outcomes of children and young adults with B-cell acute lymphoblastic leukemia given blinatumomab as last consolidation treatment before allogeneic hematopoietic stem cell transplantation
- PMID: 39479863
- PMCID: PMC11873699
- DOI: 10.3324/haematol.2024.286350
Outcomes of children and young adults with B-cell acute lymphoblastic leukemia given blinatumomab as last consolidation treatment before allogeneic hematopoietic stem cell transplantation
Abstract
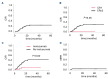
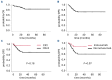
Blinatumomab has remarkable efficacy in patients with relapsed/refractory (r/r) or measurable residual disease (MRD)-positive B-cell acute lymphoblastic leukemia (B-ALL). In many patients, blinatumomab treatment is followed by allogeneic hematopoietic stem cell transplantation (HSCT). However, the influence of blinatumomab on HSCT outcomes in children and young adults (YA) remains to be fully elucidated. We conducted a single-center, retrospective analysis of patients given blinatumomab as last treatment before HSCT. Seventy-eight pediatric and YA patients were evaluated. With a median follow- up of 23.23 months, the 2-year disease-free (DFS) and overall survival (OS) probability were 72.2% and 89.2%, respectively, with a 2-year cumulative incidence of non-relapse mortality (NRM) of 2.6%. A trend toward improved 2-year DFS, but not OS, was noted in patients transplanted in first complete remission (CR1) (92.9%) compared to those in second or greater remission (CR2/3) (68.5%; P=0.18) due to a lower cumulative incidence of relapse (0% vs. 29.9%; P=0.05). Among CR2/3 patients, those receiving the sequential combination of inotuzumab and blinatumomab had a significantly lower cumulative incidence of relapse as compared to those who did not receive inotuzumab (9.5% vs. 40.4%; P=0.023). Relapse after HSCT occurred in 16 patients, all exhibiting CD19-positive blasts; ten of them received anti-CD19 chimeric antigen receptor T-cell (CAR T) therapy and two inotuzumab as salvage therapy, leading to a 2-year post-relapse OS of 52.7%. Our results indicate that HSCT following blinatumomab in children and YA with B-ALL is highly effective, being associated with low NRM and not affecting the efficacy of subsequent salvage immunotherapies, including CAR T cells.
Figures
References
-
- Lovisa F, Zecca M, Rossi B, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol. 2018;180(5):680-693. - PubMed
-
- Algeri M, Del Bufalo F, Galaverna F, Locatelli F. Current and future role of bispecific T-cell engagers in pediatric acute lymphoblastic leukemia. Expert Rev Hematol. 2018;11(12):945-956. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

