Emerging mosquito-borne flaviviruses
- PMID: 39480108
- PMCID: PMC11633211
- DOI: 10.1128/mbio.02946-24
Emerging mosquito-borne flaviviruses
Abstract
Flaviviruses comprise a genus of enveloped, positive-sense, single-stranded RNA viruses typically transmitted between susceptible and permissive hosts by arthropod vectors. Established flavivirus threats include dengue viruses (DENV), yellow fever virus (YFV), Zika virus (ZIKV), and West Nile virus (WNV), which continue to cause over 400 million infections annually and are significant global health and economic burdens. Additionally, numerous closely related but largely understudied viruses circulate in animals and can conceivably emerge in human populations. Previous flaviviruses that were recognized to have this potential include ZIKV and WNV, which only became extensively studied after causing major outbreaks in humans. More than 50 species exist within the flavivirus genus, which can be further classified as mosquito-borne, tick-borne, insect-specific, or with no known vector. Historically, many of these flaviviruses originated in Africa and have mainly affected tropical and subtropical regions due to the ecological niche of mosquitoes. However, climate change, as well as vector and host migration, has contributed to geographical expansion, thereby posing a potential risk to global populations. For the purposes of this minireview, we focus on the mosquito-borne subgroup and highlight viruses that cause significant pathology or lethality in at least one animal species and/or have demonstrated an ability to infect humans. We discuss current knowledge of these viruses, existing animal models to study their pathogenesis, and potential future directions. Emerging viruses discussed include Usutu virus (USUV), Wesselsbron virus (WSLV), Spondweni virus (SPOV), Ilheus virus (ILHV), Rocio virus (ROCV), Murray Valley encephalitis virus (MVEV), and Alfuy virus (ALFV).
Keywords: antiviral immunity; emerging viruses; flavivirus; infectious disease; species tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
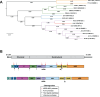
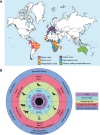
References
-
- Gould EA, de Lamballerie X, de A. Zanotto PM, Holmes EC. 2001. Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies, p 71–103. In In Advances in virus research. Academic Press. - PubMed
-
- Moureau G, Cook S, Lemey P, Nougairede A, Forrester NL, Khasnatinov M, Charrel RN, Firth AE, Gould EA, de Lamballerie X. 2015. New insights into flavivirus evolution, taxonomy and biogeographic history, extended by analysis of canonical and alternative coding sequences. PLOS ONE 10:e0117849. doi:10.1371/journal.pone.0117849 - DOI - PMC - PubMed
-
- Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, Ibe N, Akorli J, Otoo S, Mutebi J-P, Kriete AL, Ewing EG, Sang R, Gloria-Soria A, Powell JR, Baker RE, White BJ, Crawford JE, McBride CS. 2020. Climate and urbanization drive mosquito preference for humans. Curr Biol 30:3570–3579. doi:10.1016/j.cub.2020.06.092 - DOI - PMC - PubMed
-
- Foster WA, Walker ED. 2019. Chapter 15 - Mosquitoes (Culicidae), p 261–325. In Mullen GR, Durden LA (ed), Medical and veterinary Entomology, Third Edition. Academic Press.
Publication types
MeSH terms
Grants and funding
- T32GM007388, T32GM148739/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- Princeton University (PU)
- R01 AI138797, R01 AI153236, R01 AI146917, R01 AI168048, R01 AI107301, R01AI181664, U19A171401/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI153236/AI/NIAID NIH HHS/United States
- T32 GM007388/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
