Axon initial segment structure and function in health and disease
- PMID: 39480263
- PMCID: PMC12239863
- DOI: 10.1152/physrev.00030.2024
Axon initial segment structure and function in health and disease
Abstract
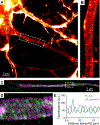
At the simplest level, neurons are structured to integrate synaptic input and perform computational transforms on that input, converting it into an action potential (AP) code. This process, converting synaptic input into AP output, typically occurs in a specialized region of the axon termed the axon initial segment (AIS). The AIS, as its name implies, is often contained to the first section of axon abutted to the soma and is home to a dizzying array of ion channels, attendant scaffolding proteins, intracellular organelles, extracellular proteins, and, in some cases, synapses. The AIS serves multiple roles as the final arbiter for determining if inputs are sufficient to evoke APs, as a gatekeeper that physically separates the somatodendritic domain from the axon proper, and as a regulator of overall neuronal excitability, dynamically tuning its size to best suit the needs of parent neurons. These complex roles have received considerable attention from experimentalists and theoreticians alike. Here, we review recent advances in our understanding of the AIS and its role in neuronal integration and polarity in health and disease.
Keywords: ankyrin; axon initial segment; excitability; ion channel; polarity.
Figures
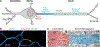
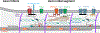

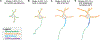
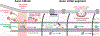



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

