Safety and tolerability of tegoprubart in patients with amyotrophic lateral sclerosis: A Phase 2A clinical trial
- PMID: 39480764
- PMCID: PMC11527214
- DOI: 10.1371/journal.pmed.1004469
Safety and tolerability of tegoprubart in patients with amyotrophic lateral sclerosis: A Phase 2A clinical trial
Abstract
Background: The interaction of CD40L and its receptor CD40 on activated T cells and B cells respectively control pro-inflammatory activation in the pathophysiology of autoimmunity and transplant rejection. Previous studies have implicated signaling pathways involving CD40L (interchangeably referred to as CD154), as well as adaptive and innate immune cell activation, in the induction of neuroinflammation in neurodegenerative diseases. This study aimed to assess the safety, tolerability, and impact on pro-inflammatory biomarker profiles of an anti CD40L antibody, tegoprubart, in individuals with amyotrophic lateral sclerosis (ALS).
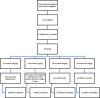
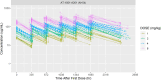
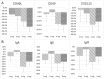
Methods and findings: In this multicenter dose-escalating open-label Phase 2A study, 54 participants with a diagnosis of ALS received 6 infusions of tegoprubart administered intravenously every 2 weeks. The study was comprised of 4 dose cohorts: 1 mg/kg, 2 mg/kg, 4 mg/kg, and 8 mg/kg. The primary endpoint of the study was safety and tolerability. Exploratory endpoints assessed the pharmacokinetics of tegoprubart as well as anti-drug antibody (ADA) responses, changes in disease progression utilizing the Revised ALS Functional Rating Scale (ALSFRS-R), CD154 target engagement, changes in pro-inflammatory biomarkers, and neurofilament light chain (NFL). Seventy subjects were screened, and 54 subjects were enrolled in the study. Forty-nine of 54 subjects completed the study (90.7%) receiving all 6 infusions of tegoprubart and completing their final follow-up visit. The most common treatment emergent adverse events (TEAEs) overall (>10%) were fatigue (25.9%), falls (22.2%), headaches (20.4%), and muscle spasms (11.1%). Mean tegoprubart plasma concentrations increased proportionally with increasing dose with a half-life of approximately 24 days. ADA titers were low and circulating levels of tegoprubart were as predicted for all cohorts. Tegoprubart demonstrated dose dependent target engagement associated and a reduction in 18 pro-inflammatory biomarkers in circulation.
Conclusions: Tegoprubart appeared to be safe and well tolerated in adults with ALS demonstrating dose-dependent reduction in pro-inflammatory chemokines and cytokines associated with ALS. These results warrant further clinical studies with sufficient power and duration to assess clinical outcomes as a potential treatment for adults with ALS.
Trial registration: Clintrials.gov ID:NCT04322149.
Copyright: © 2024 Perrin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
SPE is an employee and Director at Eledon Pharmaceuticals. MR has Honorariums: UCB Pharmaceuticals, Argenx Pharmaceuticals, Alexion Pharmaceuticals. And Grant support: UCB Pharmaceuticals, Argenx Pharmaceuticals, Alexion Pharmaceuticals, Catalyst Pharmaceuticals, Cartesian Therapeutics, Inc., Viela Bio,Momenta Pharmaceuticals, Inc, Seelos Therapeutics, Inc., PTC THERAPEUTICS, INC., Amylyx Pharmaceuticals Inc., Healey Center, NMD Pharma, Janssen Research and Development, Apellis Pharma, MedicinNova Inc, Millennium Pharma, Orion Pharma, Biohaven Pharma, Seikagaku Corporation, Mallinckrodt ARD, Inc, Cytokinetics, Inc, Grifols.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

