Discovery of PANoptosis-related signatures correlates with immune cell infiltration in psoriasis
- PMID: 39480805
- PMCID: PMC11527320
- DOI: 10.1371/journal.pone.0310362
Discovery of PANoptosis-related signatures correlates with immune cell infiltration in psoriasis
Abstract
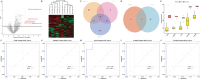
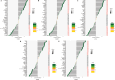
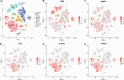
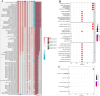
Psoriasis is an inflammatory skin disease that relapses frequently. Keratinocyte apoptosis dysregulation plays a crucial role in the pathological mechanisms of psoriasis. PANoptosis is a process with intermolecular interaction among pyroptosis, apoptosis, and necroptosis. The mechanism of PANoptosis in the occurrence and development of psoriasis is still unclear. Here we present a novel approach by identifying PANoptosis-related signatures (PANoptosis-sig) from skin tissue of psoriasis patients and healthy controls on transcriptional and protein levels. Five PANoptosis-sig (TYMP, S100A8, S100A9, NAMPT, LCN2) were identified. Enrichment analysis showed they were mainly enriched in response to leukocyte aggregation, leukocyte migration, chronic inflammatory response and IL-17 signaling pathway. Single cell transcriptome analysis showed TYMP and NAMPT were expressed in almost all cell populations, while LCN2, S100A8 and S100A9 were significantly highly expressed in keratinocyte. We then constructed predictive and diagnostic models with the PANoptosis-sig and evaluated their performance. Finally, unsupervised consensus clustering analysis was conducted to ascertain psoriasis molecular subtypes by the PANoptosis-sig. The psoriasis cohort was divided into two distinct subtypes. Immune landscape showed that the stromal score of cluster 1 was significantly higher than cluster 2, while the immune and estimate scores of cluster 2 were expressively higher than cluster 1. Cluster 1 exhibited high expression of Plasma cells, Tregs and Mast cells resting, while cluster 2 showed high expression of T cells, Macrophages M1, Dendritic cells activated, and Neutrophils in immune infiltration analysis. And cluster 2 was more sensitive to immune checkpoints. In conclusion, our findings revealed potential biomarkers and therapeutic targets for the prevention, diagnosis, and treatment of psoriasis, enhancing our understanding of the molecular mechanisms underlying PANoptosis.
Copyright: © 2024 Wu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Medovic MV, Jakovljevic VL, Zivkovic VI, Jeremic NS, Jeremic JN, Bolevich SB, et al.. Psoriasis between Autoimmunity and Oxidative Stress: Changes Induced by Different Therapeutic Approaches. Oxidative medicine and cellular longevity. 2022;2022:2249834. doi: 10.1155/2022/2249834 - DOI - PMC - PubMed
-
- Palazzo E, Morandi P, Lotti R, Saltari A, Truzzi F, Schnebert S, et al.. Notch Cooperates with Survivin to Maintain Stemness and to Stimulate Proliferation in Human Keratinocytes during Ageing. International journal of molecular sciences. 2015;16(11):26291–302. doi: 10.3390/ijms161125948 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

