The evolutionarily conserved PhLP3 is essential for sperm development in Drosophila melanogaster
- PMID: 39480878
- PMCID: PMC11527243
- DOI: 10.1371/journal.pone.0306676
The evolutionarily conserved PhLP3 is essential for sperm development in Drosophila melanogaster
Abstract
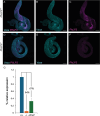
Phosducin-like proteins (PhLP) are thioredoxin domain-containing proteins that are highly conserved across unicellular and multicellular organisms. PhLP family proteins are hypothesized to function as co-chaperones in the folding of cytoskeletal proteins. Here, we present the initial molecular, biochemical, and functional characterization of CG4511 as Drosophila melanogaster PhLP3. We cloned the gene into a bacterial expression vector and produced enzymatically active recombinant PhLP3, which showed similar kinetics to previously characterized orthologues. A fly strain homozygous for a P-element insertion in the 5' UTR of the PhLP3 gene exhibited significant downregulation of PhLP3 expression. We found these male flies to be sterile. Microscopic analysis revealed altered testes morphology and impairment of spermiogenesis, leading to a lack of mature sperm. Among the most significant observations was the lack of actin cones during sperm maturation. Excision of the P-element insertion in PhLP3 restored male fertility, spermiogenesis, and seminal vesicle size. Given the high level of conservation of PhLP3, our data suggests PhLP3 may be an important regulator of sperm development across species.
Copyright: © 2024 Petit et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

