Amyloid accelerator polyphosphate fits as the mystery density in α-synuclein fibrils
- PMID: 39480879
- PMCID: PMC11527176
- DOI: 10.1371/journal.pbio.3002650
Amyloid accelerator polyphosphate fits as the mystery density in α-synuclein fibrils
Abstract
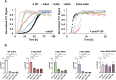
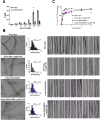
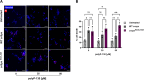
Aberrant aggregation of α-Synuclein is the pathological hallmark of a set of neurodegenerative diseases termed synucleinopathies. Recent advances in cryo-electron microscopy have led to the structural determination of the first synucleinopathy-derived α-Synuclein fibrils, which contain a non-proteinaceous, "mystery density" at the core of the protofilaments, hypothesized to be highly negatively charged. Guided by previous studies that demonstrated that polyphosphate (polyP), a universally conserved polyanion, significantly accelerates α-Synuclein fibril formation, we conducted blind docking and molecular dynamics simulation experiments to model the polyP binding site in α-Synuclein fibrils. Here, we demonstrate that our models uniformly place polyP into the lysine-rich pocket, which coordinates the mystery density in patient-derived fibrils. Subsequent in vitro studies and experiments in cells revealed that substitution of the 2 critical lysine residues K43 and K45 with alanine residues leads to a loss of all previously reported effects of polyP binding on α-Synuclein, including stimulation of fibril formation, change in filament conformation and stability as well as alleviation of cytotoxicity. In summary, our study demonstrates that polyP fits the unknown electron density present in in vivo α-Synuclein fibrils and suggests that polyP exerts its functions by neutralizing charge repulsion between neighboring lysine residues.
Copyright: © 2024 Huettemann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
UJ is a member of the PLOS Biology Editorial Board.
Figures

Update of
-
Amyloid Accelerator Polyphosphate Implicated as the Mystery Density in α-Synuclein Fibrils.bioRxiv [Preprint]. 2024 May 2:2024.05.01.592011. doi: 10.1101/2024.05.01.592011. bioRxiv. 2024. Update in: PLoS Biol. 2024 Oct 31;22(10):e3002650. doi: 10.1371/journal.pbio.3002650. PMID: 38746133 Free PMC article. Updated. Preprint.
References
-
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273(16):9443–9449. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

