A molecular mechanism for bright color variation in parrots
- PMID: 39480920
- PMCID: PMC7617403
- DOI: 10.1126/science.adp7710
A molecular mechanism for bright color variation in parrots
Abstract
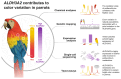
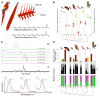
Parrots produce stunning plumage colors through unique pigments called psittacofulvins. However, the mechanism underlying their ability to generate a spectrum of vibrant yellows, reds, and greens remains enigmatic. We uncover a unifying chemical basis for a wide range of parrot plumage colors, which result from the selective deposition of red aldehyde- and yellow carboxyl-containing psittacofulvin molecules in developing feathers. Through genetic mapping, biochemical assays, and single-cell genomics, we identified a critical player in this process, the aldehyde dehydrogenase ALDH3A2, which oxidizes aldehyde psittacofulvins into carboxyl forms in late-differentiating keratinocytes during feather development. The simplicity of the underlying molecular mechanism, in which a single enzyme influences the balance of red and yellow pigments, offers an explanation for the exceptional evolutionary lability of parrot coloration.
Conflict of interest statement
RAr, SB, JB, PMA, JCC, and MC are inventors on a pending patent application related to the technology described in this work. The remaining authors declare no conflict of interest.
Figures





References
-
- Cuthill IC, Allen WL, Arbuckle K, Caspers B, Chaplin G, Hauber ME, Hill GE, Jablonski NG, Jiggins CD, Kelber A, Mappes J, et al. The biology of color. Science. 2017;357:eaan0221. - PubMed
-
- Svensson, Wong Carotenoid-based signals in behavioural ecology: a review. Behaviour. 2011;148:131–189.
-
- Amundsen T. Why are female birds ornamented? Trends Ecol Evol. 2000;15:149–155. - PubMed
-
- Hill GE. In: Bird Coloration, Volume 2: Function and Evolution. Hill GE, McGraw KJ, editors. Harvard University Press; Cambridge, MA: 2006. Female choice for ornamental coloration; pp. 137–200.
-
- Terrill RS, Shultz AJ. Feather function and the evolution of birds. Biological Reviews. 2023;98:540–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

