TGFβ2-Driven Ferritin Degradation and Subsequent Ferroptosis Underlie Salivary Gland Dysfunction in Postmenopausal Conditions
- PMID: 39481440
- PMCID: PMC11653620
- DOI: 10.1002/advs.202400660
TGFβ2-Driven Ferritin Degradation and Subsequent Ferroptosis Underlie Salivary Gland Dysfunction in Postmenopausal Conditions
Abstract
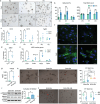
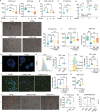
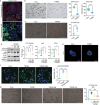
Despite the high incidence of dry mouth in postmenopausal women, its underlying mechanisms and therapeutic interventions remain underexplored. Using ovariectomized (OVX) mouse models, here this study identifies ferroptosis, an iron-dependent regulated cell death, as a central mechanism driving postmenopausal salivary gland (SG) dysfunction. In the OVX-SGs, TGFβ signaling pathway is enhanced with the aberrant TGFβ2 expression in SG mesenchymal cells. Intriguingly, TGFβ2 treatment reduces iron-storing ferritin levels, leading to lipid peroxidation and ferroptotic death in SG epithelial organoids (SGOs). Mechanistically, TGFβ2 promotes the autophagy-mediated ferritin degradation, so-called ferritinophagy. A notable overexpression of the type III TGFβ receptor (TβRIII) is found in the OVX-SGs and TGFβ2-treated SGOs, while the silencing of TβRIII mitigates the ferroptosis-mediated deleterious effects of TGFβ2 on SGOs. Finally, administration of ferroptosis inhibitor, Liproxstatin-1 (Lip-1), improves saliva secretion in OVX mice. Present findings collectively suggest a link between TGFβ signaling, ferroptosis, and SG injury, offering new therapeutic avenues for postmenopausal xerostomia.
Keywords: TGFβ2; estrogen; ferroptosis; organoids; salivary gland; xerostomia.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- 2018-R1A5A2023879/National Research Foundation of Korea by the Ministry of Science and ICT (MSIT)
- 2022-R1A2C2006697/National Research Foundation of Korea by the Ministry of Science and ICT (MSIT)
- RS-2024-00340037/National Research Foundation of Korea by the Ministry of Science and ICT (MSIT)
- RS-2024-00340063/National Research Foundation of Korea by the Ministry of Science and ICT (MSIT)
- RS-2023-00223591/Bio & Medical Technology Development Program of the National Research Foundation (NRF) by the Korean government (MSIT)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
