Alpha-1 antitrypsin inhibits pertussis toxin
- PMID: 39481600
- PMCID: PMC11683240
- DOI: 10.1016/j.jbc.2024.107950
Alpha-1 antitrypsin inhibits pertussis toxin
Abstract
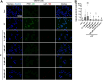
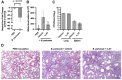
Pertussis (whooping cough) is a vaccine-preventable but re-emerging, highly infectious respiratory disease caused by Bordetella pertussis. There are currently no effective treatments for pertussis, complicating care for nonvaccinated individuals, especially newborns. Disease manifestations are predominantly caused by pertussis toxin (PT), a pivotal virulence factor classified as an ADP-ribosylating AB-type protein toxin. In this work, an unbiased approach using peptide libraries, bioassay-guided fractionation and mass spectrometry revealed α1-antitrypsin (α1AT) as a potent PT inhibitor. Biochemistry-, cell culture-, and molecular modeling-based in vitro experimentation demonstrated that the α1AT mode of action is based on blocking PT-binding to the host target cell surface. In the infant mouse model of severe pertussis, α1AT expression was reduced upon infection. Further, systemic administration of α1AT significantly reduced B. pertussis-induced leukocytosis, which is a hallmark of infant infection and major risk factor for fatal pertussis. Taken together our data demonstrates that α1AT is a novel PT inhibitor and that further evaluation and development of α1AT as a therapeutic agent for pertussis is warranted. Importantly, purified α1AT is already in use clinically as an intravenous augmentation therapy for those with genetic α1AT deficiency and could be repurposed to clinical management of pertussis.
Keywords: Bordetella pertussis; G-protein; bacterial toxin; host defense; infectious disease; inhibitor; pertussis; pertussis toxin; toxin inhibitor; whooping cough; α(1)-antitrypsin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


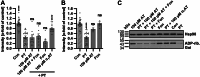








References
-
- Yeung K.H.T., Duclos P., Nelson E.A.S., Hutubessy R.C.W. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect. Dis. 2017;17:974–980. - PubMed
-
- Bagcchi S. Pertussis cases rise in Denmark. Lancet Infect. Dis. 2023;23:e469. - PubMed
-
- Dalby T. Clarifying pertussis in Denmark. Lancet Infect. Dis. 2024;24:e77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

