Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial
- PMID: 39482336
- PMCID: PMC11527888
- DOI: 10.1038/s41541-024-01003-x
Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial
Abstract
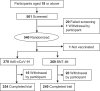
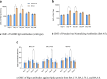
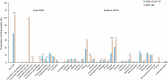
This phase 3, observer-blinded, non-inferiority randomized trial (ClinicalTrials.gov: NCT05517642), conducted from September 2022 to May 2023 at three Malaysian sites, involved 540 adults previously vaccinated with three COVID-19 doses. Participants were randomized 1:1 to receive either one dose of inhaled Recombinant COVID-19 Vaccine (Ad5-nCoV-IH) or intramuscular tozinameran (BNT-IM). The study assessed safety, vaccine efficacy (VE) and immunogenicity against SARS-CoV-2 variants. The primary outcome was the non-inferiority of anti-spike protein receptor-binding domain (S-RBD IgG) antibodies, with a 97.5% confidence interval lower limit for the geometric mean concentration (GMC) ratio >0.67. Ad5-nCoV-IH showed lower immunogenicity than BNT-IM, with a GMC ratio of 0.22 and a seroconversion rate difference of -71.91%. Adverse drug reactions (ADRs) were less frequent with Ad5-nCoV-IH (39.26%) compared to BNT-IM (64.68%). No serious vaccine-related adverse events were reported. Both vaccines had comparable efficacy against COVID-19 variants. This study was funded by Tianjin Biomedical Science and Technology Major Project.
© 2024. The Author(s).
Conflict of interest statement
T.Z., J.G., R. W., S.A, H.H., X.Y.Z, L.W., M.Y., Y.L. and V.K.C. reported being employed by CanSino Biologics Inc. which manufacture the study vaccine, during the conduct of the study and outside the submitted work. J.G. and T.Z. reported holding stock in CanSino Biologics Inc. T.O.L. reported serving as a paid senior scientific advisor to CanSino Biologics Inc. S.Ng., D.N., S.B., R.Y., N.Z., S.S.C., A.A.A., C.C.K. and, L.H.P. reported receiving research grants via Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. during the conduct of the study and outside the submitted work. R.R. and X.W. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. X.X.X and J.S.T. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. G.Y.C., A.M.N, K.Y.C, Y.L.L, W.H.W.M, M.R.M.D., W.M.R.W.A.K. and M.H.T. reported receiving research grants from Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. No other disclosures were reported.
Figures
References
-
- Halperin, S. A. et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet399, 237–248 (2022). - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

