The MCP-3/Ccr3 axis contributes to increased bone mass by affecting osteoblast and osteoclast differentiation
- PMID: 39482538
- PMCID: PMC11612511
- DOI: 10.1038/s12276-024-01344-6
The MCP-3/Ccr3 axis contributes to increased bone mass by affecting osteoblast and osteoclast differentiation
Abstract
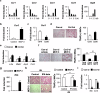
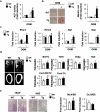
Several CC subfamily chemokines have been reported to regulate bone metabolism by affecting osteoblast or osteoclast differentiation. However, the role of monocyte chemotactic protein 3 (MCP-3), a CC chemokine, in bone remodeling is not well understood. Here, we show that MCP-3 regulates bone remodeling by promoting osteoblast differentiation and inhibiting osteoclast differentiation. In a Ccr3-dependent manner, MCP-3 promoted osteoblast differentiation by stimulating p38 phosphorylation and suppressed osteoclast differentiation by upregulating interferon beta. MCP-3 increased bone morphogenetic protein 2-induced ectopic bone formation, and mice with MCP-3-overexpressing osteoblast precursor cells presented increased bone mass. Moreover, MCP-3 exhibited therapeutic effects by abrogating receptor activator of nuclear factor kappa-B ligand-induced bone loss. Therefore, MCP-3 has therapeutic potential for diseases involving bone loss due to its positive role in osteoblast differentiation and negative role in osteoclast differentiation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Kim, K., Han, J. E., Lee, K. B. & Kim, N. LIM homeobox transcription factor 1-β expression is upregulated in patients with osteolysis after total ankle arthroplasty and inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation in vitro. J. Bone Metab.29, 165–174 (2022). - PMC - PubMed
-
- Wintges, K. et al. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J. Bone Min. Res28, 2070–2080 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

