The transcription factor BBX regulates phosphate homeostasis through the modulation of FGF23
- PMID: 39482539
- PMCID: PMC11612488
- DOI: 10.1038/s12276-024-01341-9
The transcription factor BBX regulates phosphate homeostasis through the modulation of FGF23
Abstract
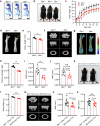
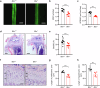
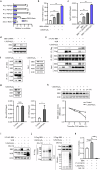
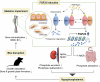
Fibroblast growth factor 23 (FGF23) plays an important role in phosphate homeostasis, and increased FGF23 levels result in hypophosphatemia; however, the molecular mechanism underlying increased FGF23 expression has not been fully elucidated. In this study, we found that mice lacking the bobby sox homolog (Bbx-/-) presented increased FGF23 expression and low phosphate levels in the serum and skeletal abnormalities such as a low bone mineral density (BMD) and bone volume (BV), as well as short and weak bones associated with low bone formation. Osteocyte-specific deletion of Bbx using Dmp-1-Cre resulted in similar skeletal abnormalities, elevated serum FGF23 levels, and reduced serum phosphate levels. In Bbx-/- mice, the expression of sodium phosphate cotransporter 2a (Npt2a) and Npt2c in the kidney and Npt2b in the small intestine, which are negatively regulated by FGF23, was downregulated, leading to phosphate excretion/wasting and malabsorption. An in vitro Fgf23 promoter analysis revealed that 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)-induced transactivation of the Fgf23 promoter was significantly inhibited by BBX overexpression, whereas it was increased following Bbx knockdown. Interestingly, 1,25(OH)2D3 induced an interaction of the 1,25(OH)2D3 receptor (VDR) with BBX and downregulated BBX protein levels. Cycloheximide (CHX) only partially downregulated BBX protein levels, indicating that 1,25(OH)2D3 regulates BBX protein stability. Furthermore, the ubiquitination of BBX followed by proteasomal degradation was required for the increase in Fgf23 expression induced by 1,25(OH)2D3. Collectively, our data demonstrate that BBX negatively regulates Fgf23 expression, and consequently, the ubiquitin-dependent proteasomal degradation of BBX is required for FGF23 expression, thereby regulating phosphate homeostasis and bone development in mice.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Subramanian, R. & Khardori, R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine79, 1–8 (2000). - PubMed
-
- Florenzano, P. et al. Approach to patients with hypophosphataemia. Lancet Diab. Endocrinol.8, 163–174 (2020). - PubMed
-
- Torres, P. A. & De Brauwere, D. P. Three feedback loops precisely regulating serum phosphate concentration. Kidney Int.80, 443–445 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

