The European reference network for metabolic diseases (MetabERN) clinical pathway recommendations for Pompe disease (acid maltase deficiency, glycogen storage disease type II)
- PMID: 39482698
- PMCID: PMC11529438
- DOI: 10.1186/s13023-024-03373-w
The European reference network for metabolic diseases (MetabERN) clinical pathway recommendations for Pompe disease (acid maltase deficiency, glycogen storage disease type II)
Abstract
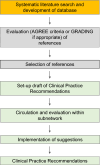
Clinical pathway recommendations (CPR) are based on existing guidelines and deliver a short overview on how to deal with a specific diagnosis, resulting therapy and follow-up. In this paper we propose a methodology for developing CPRs for Pompe disease, a metabolic myopathy caused by deficiency of lysosomal acid alpha-glucosidase. The CPR document was developed within the activities of the MetabERN, a non-profit European Reference Network for Metabolic Diseases established by the European Union. A working group was selected among members of the MetabERN lysosomal storage disease subnetwork, with specific expertise in the care of Pompe disease, and patient support group representatives. The working strategy was based on a systematic literature search to develop a database, followed by quality assessment of the studies selected from the literature, and by the development of the CPR document according to a matrix provided by MetabERN. Quality assessment of the literature and collection of citations was conducted according to the AGREE II criteria and Grading of Recommendations, Assessment, Development and Evaluation methodology. General aspects were addressed in the document, including pathophysiology, genetics, frequency, classification, manifestations and clinical approach, laboratory diagnosis and multidisciplinary evaluation, therapy and supportive measures, follow-up, monitoring, and pregnancy. The CPR document that was developed was intended to be a concise and easy-to-use tool for standardization of care for patients among the healthcare providers that are members of the network or are involved in the care for Pompe disease patients.
Keywords: Acid alpha-glucosidase deficiency; Acid maltase deficiency; Glycogen storage disease (GSD) type II; Lysosomal storage disease; Pompe disease.
© 2024. The Author(s).
Conflict of interest statement
GP has received funding for research and clinical trials and advisory fees from Sanofi-Genzyme, Amicus Therapeutics, Orchard, Synageva/Alexion, BioMarin, Denali, Takeda. SF has received honoraria, consulting fees, speaker fees and travel reimbursement. From Sanofi, Amicus, Alexion, Chiesi. MJG has received educational and research grants from Sanofi Genzyme. PRTA received grant/research support from Takeda, and speaker/travel. honoraria from Takeda, Sanofi-Genzyme, BioMarin, Ultragenyx, Alexion, Amicus Therapeutics, and Chiesi.. OA has received educational/research grants from Shire Human Genetic Therapies/Takeda and travel/accommodation support for conferences from Shire Human Genetic. Therapies/Takeda, Amicus and Sanofi Genzyme. MAD received travel grants for scientific meetings and honoraria for speaking. engagements from Shire International, Sanofi Genzyme and BioMarin. MS has received honoraria, research and travel grants from Alexion, BioMarin. Pharmaceutical Inc., Chiesi, Sanofi Genzyme, Shire, Ultragenix and Sangamo. NAMEvdeB has received consulting fees and travel reimbursement from Sanofi andAmicus Therapeutics and received funding for research, clinical trials, and advisory fees from Sanofi. MDTR has received consulting fees and speaker honoraria, travel expenses, and congress fees from Biomarin, Sanofi Genzyme, Takeda,and has participated in trials sponsored by Orphazyme, Takeda, Vtesse-Sucampo Mallinckrodt). DPG has received consulting honoraria from Chiesi, Idorsia Pharmaceuticals, Sanofi and Takeda, and speaker honoraria and travel support from Sanofi and Takeda. HH reports advisory board fees, speaker fee, and a clinical trial agreement from BioMarin. JMPvdH received funding for research, clinical trials, and advisory fees from Sanofi-Genzyme, Amicus Therapeutics, BioMarin, Sarepta, Denali, Takeda and Chiesi. ATvdP has received grant for clinical trials conduction from Amicus Therapeutics andSanofi-Genzyme. MA, FA, AV, AT, VG, AZ, AH, BKW have no conflicts of interest to declare.
Figures
References
-
- van der Ploeg AT, Laforet P. Pompe disease. In: Hollak C, Lachmann R, editors. Inherited metabolic disease in adults: a clinical guide. Oxford University Press; 2016. p. 353–8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

