Paclitaxel- or sirolimus-coated balloons used for ArterioVEnous fistulas-2 (PAVE-2): study protocol for a randomised controlled trial to determine the efficacy of paclitaxel- or sirolimus-coated balloons in arteriovenous fistulas used for haemodialysis
- PMID: 39482722
- PMCID: PMC11529252
- DOI: 10.1186/s13063-024-08502-1
Paclitaxel- or sirolimus-coated balloons used for ArterioVEnous fistulas-2 (PAVE-2): study protocol for a randomised controlled trial to determine the efficacy of paclitaxel- or sirolimus-coated balloons in arteriovenous fistulas used for haemodialysis
Abstract
Background: In view of the conflicting results from previous studies, the benefit of paclitaxel-coated balloons for arteriovenous fistulas is uncertain and equipoise remains. Although an industry-led trial testing the efficacy of sirolimus-coated balloons in AVFs is in progress, the benefit of sirolimus-coated balloons for arteriovenous fistulas is currently unknown. The purpose of this trial is to compare the efficacy of additional paclitaxel-coated or sirolimus-coated balloons on outcomes after a plain balloon fistuloplasty to preserve the patency of arteriovenous fistulae used for haemodialysis.
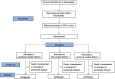
Methods: The study design is a multicentre randomised controlled trial. Following a successful plain balloon fistuloplasty, participants will be randomised to further treatment with a paclitaxel-coated balloon, a sirolimus-coated balloon, or an uncoated control balloon. We will recruit 642 patients, each with one or two treatment segments, over a 3-year period. Patients will remain in the trial and be followed up for 1 year. The primary endpoint is time to loss of treatment segment primary patency. Cox-proportional hazards models will be used to estimate hazard ratios for the time to loss of treatment segment primary patency for each treatment group relative to the control group. Analysis of the primary endpoint will be based on treatment segments rather than participants and a shared frailty will be estimated to account for the clustering of treatment segments within patients. Secondary endpoints are time to loss of primary patency at any treatment segment; time to end of access circuit primary patency; time to AVF abandonment; number of radiological or surgical interventions; adverse events; intima-media thickness and degree of stenosis at 3 months on ultrasound; and patient quality of life assessed by EQ-5D-5L and VASQoL.
Discussion: The three-armed design in this proposal will provide an answer on the efficacy of both paclitaxel- and sirolimus-coated balloons in the same trial. This trial is likely to provide a clear answer regarding the efficacy of drug-coated balloons for arteriovenous fistulas.
Trial registration: ISRCTN ISRCTN40182296. Registered on 4 August 2023.
Keywords: Angioplasty; Arteriovenous fistula; Fistuloplasty; Haemodialysis; Paclitaxel; Sirolimus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- The Vascular Priority Setting Partnership. Setting the agenda for UK vascular research. JVascSocGBIrel. 2021;1(1):1–31.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical