Impact of Side Chains in 1-n-Alkylimidazolium Ionomers on Cu-Catalyzed Electrochemical CO2 Reduction
- PMID: 39482894
- PMCID: PMC11653625
- DOI: 10.1002/advs.202406281
Impact of Side Chains in 1-n-Alkylimidazolium Ionomers on Cu-Catalyzed Electrochemical CO2 Reduction
Abstract
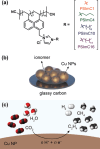
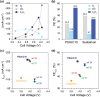
This study presents the impact of the side chains in 1-n-alkylimidazolium ionomers with varying side chain lengths (CnH2n+1 where n = 1, 4, 10, 16) on Cu-catalyzed electrochemical CO2 reduction reaction (CO2RR). Longer side chains suppress the H2 and CH4 formation, with the n-hexadecyl ionomer (n = 16) showing the greatest reduction in kinetics by up to 56.5% and 60.0%, respectively. On the other hand, C2H4 production demonstrates optimal Faradaic efficiency with the n-decyl ionomer (n = 10), a substantial increase of 59.9% compared to its methyl analog (n = 1). Through a combination of density functional theory calculations and material characterization, it is revealed that the engineering of the side chains effectively modulates the thermodynamic stability of key intermediates, thus influencing the selectivity of both CO2RR and hydrogen evolution reaction. Moreover, ionomer engineering enables industrially relevant partial current density of -209.5 mA cm-2 and a Faradaic efficiency of 52.4% for C2H4 production at 3.95 V, even with a moderately active Cu catalyst, outperforming previous benchmarks and allowing for further improvement through catalyst engineering. This study underscores the critical role of ionomers in CO2RR, providing insights into their optimal design for sustainable chemical synthesis.
Keywords: CO2 reduction; Cu catalyst; DFT; binder; ionomer.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

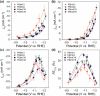


 and (d) versus cell voltage. The results from this study are indicated as PSImC10 and Sustainion. For comparative analysis, literature data were extracted from studies using Cu catalysts of moderate catalytic activity (i.e., commercial Cu nanoparticles or sputtered Cu). Symbols: square (PSImC10), pentagon (Sustainion), inverted triangle (Nafion), and circle (PTFE).
and (d) versus cell voltage. The results from this study are indicated as PSImC10 and Sustainion. For comparative analysis, literature data were extracted from studies using Cu catalysts of moderate catalytic activity (i.e., commercial Cu nanoparticles or sputtered Cu). Symbols: square (PSImC10), pentagon (Sustainion), inverted triangle (Nafion), and circle (PTFE).References
-
- Li L., Li X., Sun Y., Xie Y., Chem. Soc. Rev. 2022, 51, 1234. - PubMed
-
- Lee W. H., Kim K., Koh J. H., Lee D. K., Won D. H., Oh H.‐S., Lee U., Min B. K., Nano Energy 2023, 110, 108373.
-
- Nabil S. K., McCoy S., Kibria M. G., Green Chem. 2021, 23, 867.
-
- Hori Y., Kikuchi K., Suzuki S., Chem. Lett. 1985, 14, 1695.
-
- Nitopi S., Bertheussen E., Scott S. B., Liu X., Engstfeld A. K., Horch S., Seger B., Stephens I. E. L., Chan K., Hahn C., Nørskov J. K., Jaramillo T. F., Chorkendorff I., Chem. Rev. 2019, 119, 7610. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
