Morphological phenotyping of the aging cochlea in inbred C57BL/6N and outbred CD1 mouse strains
- PMID: 39482905
- PMCID: PMC11709085
- DOI: 10.1111/acel.14362
Morphological phenotyping of the aging cochlea in inbred C57BL/6N and outbred CD1 mouse strains
Abstract
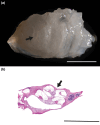
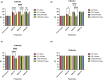
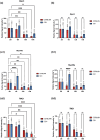
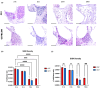
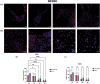
Morphological mouse phenotyping plays a pivotal role in the translational setting and even more in the area of auditory research, where mouse is a central model organism due to the evolutionary genetic relationship and morpho-functional analogies with the human auditory system. However, some results obtained in murine models cannot be translated to humans due to the inadequate description of experimental conditions underlying poor reproducibility. We approach the characterization of the aging process of the mouse cochlea in animals up to 18 months of age belonging to two of the most used outbred (CD1) and inbred (C57BL/6N) strains. Striving to reduce any environmental variable we performed our study compliantly to the ARRIVE guidelines. We integrated instrumental data (auditory brainstem response test), with morphological analyses to correlate functional discrepancies to morphological changes and track the differences in the evolution of sensorineural hearing loss in the two strains. We featured the localization of Gipc3, Myosin VIIa, and TMC1 in hair cells of the Corti organ as well as NF 200 and the density of type I neuron in the spiral ganglion. We outlined age-related hearing loss (ARHL) in both strains, and a clear drop in the selected marker localization. However, in CD1 we detected a different trend allowing the identification of potential strain-specific mechanisms, namely an increase in myosin VIIa in 6 months aging mice in comparison to 2 months old animals. Our findings represent an asset to investigate the strain-dependent physiological trigger of ARHL providing new insights in the translational area.
Keywords: aging cochlea; animal models; morphological phenotyping; mouse strains; sensorineural hearing loss.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
All authors disclosed no conflicts of interest.
Figures

References
-
- Charizopoulou, N. , Lelli, A. , Schraders, M. , Ray, K. , Hildebrand, M. S. , Ramesh, A. , Srisailapathy, C. R. S. , Oostrik, J. , Admiraal, R. J. C. , Neely, H. R. , Latoche, J. R. , Smith, R. J. H. , Northup, J. K. , Kremer, H. , Holt, J. R. , & Noben‐Trauth, K. (2011). Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nature Communications, 2, 201. 10.1038/ncomms1200 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

